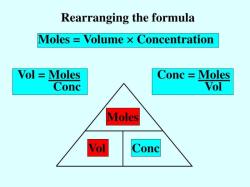
How do you calculate mole?
To calculate the number of moles (often denoted as "n") in a given sample of a substance, you can use the following formula:
n = m / M
Where:
- n is the number of moles.
- m is the mass of the substance in grams.
- M is the molar mass of the substance in grams per mole.
Here's a step-by-step guide on how to calculate moles:
Determine the Mass: First, measure or find the mass of the substance you want to calculate the moles for. Make sure it is in grams (g).
Find the Molar Mass: Look up the molar mass of the substance. The molar mass is the mass of one mole of that substance and is typically expressed in g/mol. You can find the molar mass on the periodic table for elements or by adding up the atomic masses of the atoms in a compound.
Use the Formula: Plug the mass (in grams) and the molar mass (in g/mol) into the formula:
n = m / M
Calculate the Moles: Divide the mass (in grams) by the molar mass (in g/mol) to find the number of moles.
Example:If you have 45 grams of water (H2O), you can calculate the moles as follows:
n = 45 g / (2 g/mol (for H) + 16 g/mol (for O)) = 45 g / 18 g/mol = 2.5 moles of water.
This calculation is fundamental in chemistry because it allows you to relate the mass of a substance to the number of atoms or molecules it contains, making it a crucial concept for stoichiometry, chemical reactions, and other chemical calculations.
How to calculate the number of moles in a chemical sample?
To calculate the number of moles in a chemical sample, you can use the following formula:
Number of moles = Mass of sample / Molar mass of sample
The molar mass of a substance is the mass in grams of one mole of that substance. It is calculated by adding up the atomic masses of all the atoms in the substance's chemical formula.
For example, to calculate the number of moles in a 10.0 gram sample of water (H2O), you would use the following steps:
- Find the molar mass of water: Molar mass of H2O = (2 * 1.008 amu) + (1 * 15.999 amu) = 18.015 amu
- Divide the mass of the sample by the molar mass of water: Number of moles of water = 10.0 g / 18.015 g/mol = 0.555 mol
What is the formula for mole calculation, and how is it used?
The formula for mole calculation is:
Number of moles = Mass of sample / Molar mass of sample
This formula is used to calculate the number of moles in a chemical sample. The number of moles is a useful unit of measurement in chemistry because it allows us to compare the number of particles in different samples of matter.
For example, we can use the mole concept to calculate the amount of reactant needed to produce a certain amount of product in a chemical reaction. We can also use the mole concept to calculate the concentration of a solution.
Can you explain the relationship between moles, mass, and molar mass?
One mole of any substance contains the same number of particles, which is approximately 6.022 x 10^23 particles. This number is known as Avogadro's number.
The molar mass of a substance is the mass in grams of one mole of that substance. It is calculated by adding up the atomic masses of all the atoms in the substance's chemical formula.
The relationship between moles, mass, and molar mass can be expressed by the following equation:
Number of moles = Mass of sample / Molar mass of sample
This equation can be used to calculate the number of moles in a chemical sample, the mass of a chemical sample, or the molar mass of a substance.
Are there online tools or calculators for mole calculations?
Yes, there are many online tools and calculators for mole calculations. Some popular options include:
- Chembook Mole Calculator: https://www.inchcalculator.com/mole-calculator/
- Stoichiometry Calculator: https://www.chemicalaid.com/tools/reactionstoichiometry.php?hl=en
- Molar Mass Calculator: https://www.lenntech.com/calculators/molecular/molecular-weight-calculator.htm
These tools and calculators can be used to perform a variety of mole calculations, such as calculating the number of moles in a sample, the mass of a sample, or the molar mass of a substance.
What is the significance of mole calculations in chemistry?
Mole calculations are significant in chemistry because they allow us to compare the number of particles in different samples of matter. This is important for understanding and predicting the behavior of matter.
For example, mole calculations can be used to:
- Calculate the amount of reactant needed to produce a certain amount of product in a chemical reaction
- Calculate the concentration of a solution
- Calculate the mass of a gas given its volume and temperature
- Calculate the number of atoms in a sample of a solid
Mole calculations are also used in a variety of other chemistry applications, such as analytical chemistry, physical chemistry, and organic chemistry.
Overall, mole calculations are an essential tool for chemists. They allow us to understand and predict the behavior of matter in a variety of situations.