Is millimolar moles per milliliter?
Millimolar (mM) and moles per milliliter (mol/mL) are related units used to express concentration, but they are not the same. They measure different aspects of concentration:
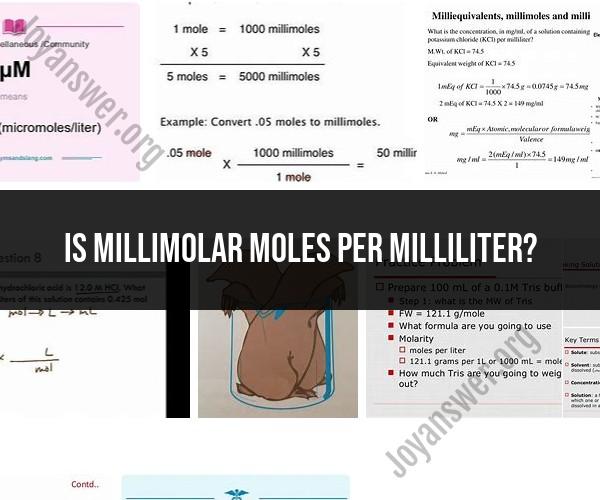
Millimolar (mM): Millimolar is a unit of concentration used to express the number of millimoles (1/1,000th of a mole) of a substance present in one liter (1,000 milliliters) of solution. It is commonly used in chemistry and biochemistry to represent the concentration of a solute in a solution.
Moles per Milliliter (mol/mL): Moles per milliliter is a measure of the concentration of a substance in terms of the number of moles present in one milliliter of a solution. It is essentially the same as molarity (M), which represents the number of moles of solute per liter of solution. Mol/mL is not a commonly used unit in scientific literature; instead, molarity (moles per liter) is the more widely recognized concentration unit.
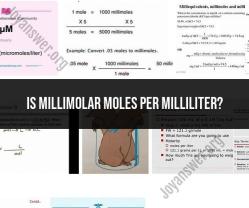
To convert from millimolar (mM) to moles per milliliter (mol/mL), you would need to know the volume of the solution in milliliters and then perform the conversion based on the following relationship:
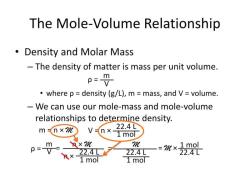
So, to convert from millimolar to moles per milliliter, you multiply the millimolar concentration by 0.001. For example, if you have a solution with a concentration of 10 mM, it is equivalent to 0.01 mol/mL.
Millimolar and Moles per Milliliter: Deciphering Concentration Units
Millimolar (mM) and moles per milliliter (mol/mL) are both units of concentration. Concentration is a measure of the amount of a solute dissolved in a solution. The solute is the substance that is being dissolved, and the solution is the mixture of the solute and the solvent. The solvent is the substance that the solute is dissolved in.
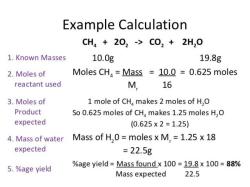
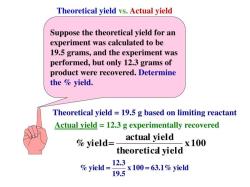
Millimolar is a unit of concentration that is used in chemistry and biochemistry. It is defined as the number of millimoles of a solute per liter of solution. A millimole is one-thousandth of a mole. One mole is equal to the mass of Avogadro's number of particles of a substance. Avogadro's number is approximately 6.022 x 10^23 particles.
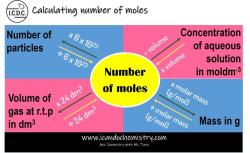
Moles per milliliter (mol/mL) is another unit of concentration. It is defined as the number of moles of a solute per milliliter of solution.
The following table shows the relationship between millimolar and moles per milliliter:
| Unit | Definition |
|---|---|
| Millimolar (mM) | Millimoles of solute per liter of solution |
| Moles per milliliter (mol/mL) | Moles of solute per milliliter of solution |
To convert from mM to mol/mL, multiply by 0.001. To convert from mol/mL to mM, multiply by 1000.
Millimolar (mM): A Measure of Concentration in Chemistry
Millimolar is a useful unit of concentration in chemistry because it is easy to calculate and interpret. For example, a 1 mM solution of a substance contains 1 millimole of the substance per liter of solution. This means that there are 1 millimole of the substance in every 1000 milliliters of solution.
Millimolar concentration is often used in biochemistry because it is a convenient way to measure the concentration of biological molecules, such as enzymes and proteins. For example, the concentration of glucose in human blood is typically around 5 mM.
Understanding Millimolar (mM) and Its Application in Science
Millimolar is a useful unit of concentration in many areas of science, including:
- Chemistry: Millimolar concentration is used to measure the concentration of solutions in chemistry. For example, the concentration of a reagent used in a chemical reaction is often expressed in millimolar.
- Biochemistry: Millimolar concentration is used to measure the concentration of biological molecules in biochemistry. For example, the concentration of enzymes and proteins in cells is often expressed in millimolar.
- Pharmacology: Millimolar concentration is used to measure the concentration of drugs in pharmacology. For example, the concentration of a drug in a patient's bloodstream is often expressed in millimolar.
Overall, millimolar is a versatile unit of concentration that is used in many different areas of science. It is a convenient way to measure the concentration of solutions, biological molecules, and drugs.