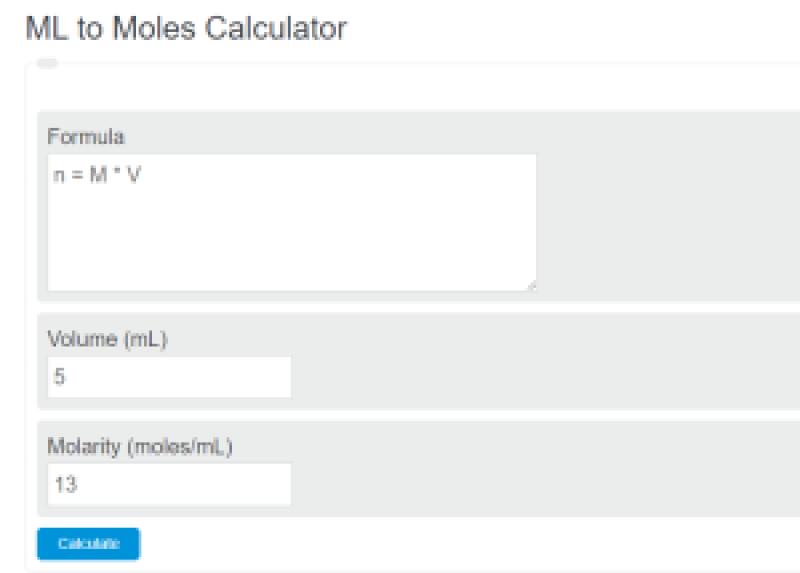
How many moles per mL?
To determine the number of moles per milliliter (mL) in a solution, you need to know the concentration of the solution, which is usually expressed in terms of molarity (moles per liter). The formula for molarity (M) is:
If you want to find the number of moles per milliliter, you need to convert the volume from milliliters to liters. The conversion factor between milliliters and liters is .
So, the formula to find moles per milliliter () is:
Here are the steps to calculate moles per milliliter:
Determine the Number of Moles (moles of solute):
- This is the amount of solute present in the solution.
Determine the Volume of the Solution in Milliliters:
- This is the total volume of the solution.
Convert Volume to Liters:
- Divide the volume in milliliters by 1000 to convert it to liters.
Apply the Formula:
- Plug the values of moles and volume in liters into the formula.
Perform the Calculation:
- Calculate the result to find the moles per milliliter.
As an example, if you have 2 moles of solute in a solution with a volume of 500 mL:
So, in this example, the solution has a concentration of 4 moles per liter, which is equivalent to 4 moles per milliliter when expressed in terms of volume.
Concentration calculation: How many moles per mL?
Concentration is a crucial concept in chemistry that represents the amount of a solute dissolved in a solvent. It is often expressed in various units, but one common unit is moles per milliliter (mol/mL). This unit indicates the number of moles of solute present in one milliliter of solution.
Understanding and calculating the concentration of a solution in moles per milliliter
The concentration of a solution in moles per milliliter can be calculated using the following formula:
Concentration (mol/mL) = moles of solute / volume of solution (mL)
For example, if you have 0.1 moles of sodium chloride (NaCl) dissolved in 100 mL of water, the concentration of the solution would be:
Concentration (mol/mL) = 0.1 moles / 100 mL = 0.001 mol/mL
This indicates that there are 0.001 moles of NaCl present in each milliliter of the solution.
Tips for chemistry students and researchers in interpreting and using concentration units
Here are some tips for chemistry students and researchers in interpreting and using concentration units:
Identify the solute and solvent: Clearly distinguish between the solute (the substance being dissolved) and the solvent (the substance in which the solute is dissolved).
Use consistent units: Ensure that the units for moles and volume are consistent, such as moles in grams and volume in milliliters or liters.
Be aware of different concentration units: Familiarize yourself with other concentration units, such as molarity (M) and mass per volume (g/mL), and their relationships to moles per milliliter.
Interpret concentration in context: Understand the significance of concentration in the context of chemical reactions, solution preparation, and analytical techniques.
Concentration is a fundamental concept in chemistry and plays a vital role in various fields, including analytical chemistry, pharmaceutical science, and environmental science. By understanding and using concentration units effectively, chemists can perform accurate calculations, interpret experimental data, and design experiments