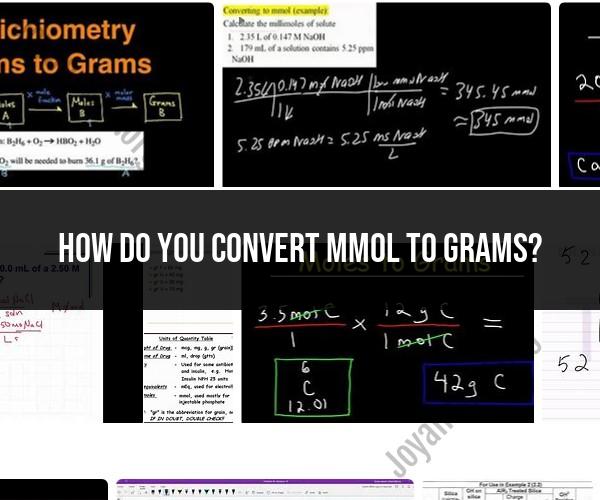
How do you convert mmol to grams?
To convert millimoles (mmol) to grams (g), you need to know the molecular mass (molar mass) of the substance you are working with. The molecular mass is typically expressed in grams per mole (g/mol). Once you have the molecular mass, you can use the following formula to perform the conversion:
Here's how you can convert millimoles to grams using this formula:
Determine the molecular mass of the substance for which you want to perform the conversion. You can find the molecular mass on the periodic table or by adding up the atomic masses of all the atoms in the chemical formula of the substance.
Multiply the number of millimoles (mmol) by the molecular mass (g/mol) to find the mass in grams (g).
For example, let's say you have 5 mmol of glucose (C6H12O6). The molecular mass of glucose is approximately 180.16 g/mol. To convert the millimoles to grams:
So, 5 mmol of glucose is equal to 900.8 grams.
Simplifying Chemistry: Converting mmol to Grams
Converting millimoles (mmol) to grams (g) is a common task in chemistry. It is necessary when preparing solutions, calculating the amount of reactant needed in a chemical reaction, or determining the product yield.
The conversion process is straightforward, but it requires knowing the molar mass of the substance being converted. The molar mass is the mass of one mole of a substance. It is expressed in grams per mole (g/mol).
The Conversion Process: Millimoles to Grams in Chemistry
To convert millimoles to grams, you can use the following formula:
Grams = Millimoles * Molar mass
For example, to convert 10 mmol of glucose to grams, you would use the following calculation:
Grams = 10 mmol * 180.16 g/mol = 1801.6 mg
The molar mass of glucose is 180.16 g/mol.
Using Molar Mass to Convert mmol to Grams
You can find the molar mass of any substance in a chemistry reference book or online. Once you know the molar mass, you can use the formula above to convert millimoles to grams.
Here is another example:
To convert 5 mmol of sodium chloride (NaCl) to grams, you would use the following calculation:
Grams = 5 mmol * 58.44 g/mol = 292.2 mg
The molar mass of sodium chloride is 58.44 g/mol.
Conclusion
Converting millimoles to grams is a simple process, but it requires knowing the molar mass of the substance being converted. You can find the molar mass of any substance in a chemistry reference book or online.