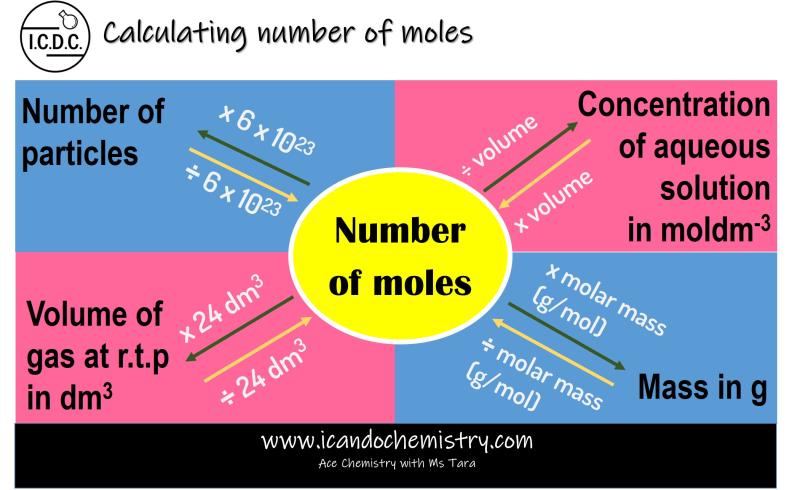
How do you calculate the number of moles in chemistry?
To calculate the number of moles in chemistry, you can use the formula:
Here's a step-by-step guide on how to calculate the number of moles:
Determine the Mass:
- Find the mass of the substance in grams. This could be given in the problem statement, provided in a lab experiment, or obtained from measurements.
Identify the Substance:
- Determine the identity of the substance for which you want to calculate moles. You will need to know the molar mass of the substance.
Find the Molar Mass:
- Look up the molar mass of the substance on the periodic table. The molar mass is the mass of one mole of the substance and is expressed in grams per mole (g/mol).
Use the Formula:
- Apply the formula:
Perform the Calculation:
- Divide the mass in grams by the molar mass to obtain the number of moles.
Example:
Let's say you have 60 grams of sodium chloride (NaCl). The molar mass of NaCl is approximately 58.44 g/mol.
The result would be approximately 1.03 moles of NaCl.
Remember the following points:
Ensure that your units are consistent. If the mass is given in grams, the molar mass should also be in grams per mole.
Use accurate molar masses from the periodic table. Round the molar mass to the appropriate number of significant figures.
If you are working with a compound with more than one element, sum the molar masses of all the elements in the compound to get the overall molar mass.
Calculating moles is a fundamental concept in chemistry and is often used in stoichiometry and other chemical calculations.
1. Stoichiometry Basics: How to Calculate the Number of Moles in Chemistry
Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between substances involved in chemical reactions. One of the essential aspects of stoichiometry is understanding how to calculate the number of moles in chemical reactions.
The mole is the SI unit of amount of substance, representing one gram of a substance's molecular weight. It is a crucial unit for performing calculations involving chemical reactions, conversions between mass and moles, and determining the amount of a reactant or product involved in a reaction.
Calculating the Number of Moles from Mass
To calculate the number of moles from mass, you can use the following formula:
moles = mass / molar mass
where:
- moles is the number of moles of the substance
- mass is the mass of the substance in grams (g)
- molar mass is the mass of one mole of the substance in grams per mole (g/mol)
The molar mass of a substance can be calculated by summing the atomic masses of all the atoms in its molecular formula. For example, the molar mass of sodium chloride (NaCl) is calculated as follows:
molar mass of NaCl = atomic mass of Na + atomic mass of Cl
molar mass of NaCl = 22.99 g/mol + 35.45 g/mol
molar mass of NaCl = 58.44 g/mol
Therefore, if you have 10 grams of NaCl, you can calculate the number of moles as follows:
moles of NaCl = 10 g / 58.44 g/mol
moles of NaCl = 0.171 moles
Calculating the Number of Moles from Volume
For gases, the number of moles can also be calculated from volume using the ideal gas law:
PV = nRT
where:
- P is the pressure of the gas in atmospheres (atm)
- V is the volume of the gas in liters (L)
- n is the number of moles of the gas
- R is the ideal gas constant, which is 0.0821 L·atm/mol·K
- T is the temperature of the gas in Kelvin (K)
Overview of stoichiometry and methods for calculating moles in chemical reactions
Stoichiometry plays a crucial role in understanding and predicting the outcome of chemical reactions. By balancing chemical equations, one can determine the mole ratios between reactants and products. These mole ratios can then be used to calculate the amount of a reactant or product needed or produced in a reaction.
Balanced Chemical Equations
A balanced chemical equation represents the chemical reaction between reactants and products, where the coefficients represent the number of moles of each substance involved. For example, the balanced equation for the combustion of methane is:
CH₄ + 2O₂ → CO₂ + 2H₂O
This equation indicates that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
Methods for Calculating Moles in Chemical Reactions
Once you have a balanced chemical equation, you can use the following methods to calculate the moles of reactants or products:
Mass-to-mole conversions: Use the molar mass to convert mass to moles and vice versa.
Mole ratios from balanced equations: Use the coefficients from the balanced equation to determine the mole ratios between reactants and products.
Limiting reactant and stoichiometric calculations: Identify the limiting reactant in a reaction and use mole ratios to determine the theoretical yield of products.
Tips for students and scientists in mastering mole calculations in chemistry
Mastering mole calculations in chemistry requires a strong understanding of the mole concept, balanced chemical equations, and stoichiometric principles. Here are some tips for students and scientists to improve their mole calculations:
Practice with basic mole conversions: Get comfortable converting between grams and moles for various substances.
Balance chemical equations accurately: Balancing chemical equations is crucial for determining mole ratios.
Understand the concept of limiting reactant: Identify the limiting reactant and use it to calculate the theoretical yield of products.
Use dimensional analysis: Dimensional analysis can help organize calculations and avoid errors.
Seek help when needed: Don't hesitate to ask for assistance from instructors, tutors, or colleagues if you encounter difficulties.