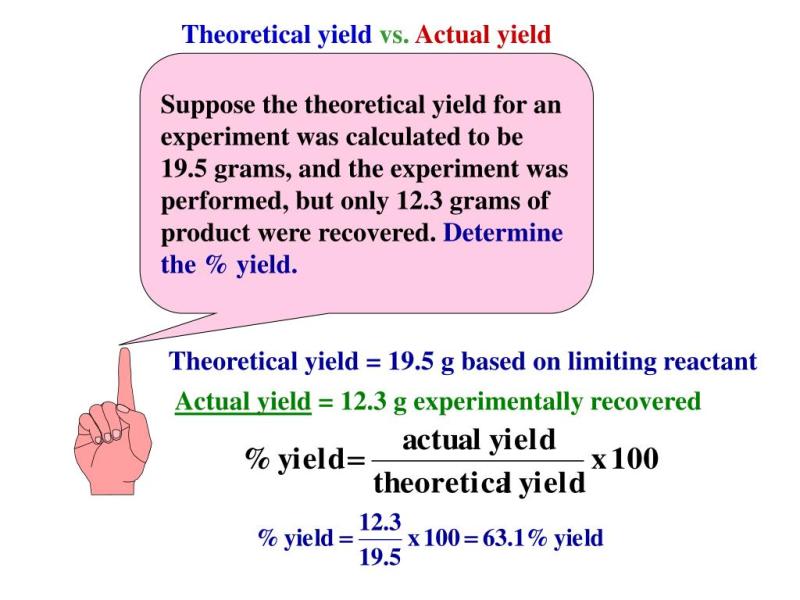
Can an actual yield ever exceed a theoretical yield?
In theory, the actual yield of a chemical reaction should not exceed the theoretical yield. The theoretical yield is the maximum amount of product that can be formed based on stoichiometry and the balanced chemical equation. It represents the ideal or perfect conditions where all reactants are converted to products with 100% efficiency.
However, in real-world laboratory or industrial settings, it's common for the actual yield to be less than the theoretical yield. This can be due to various factors such as incomplete reactions, side reactions, impurities, loss during handling or transfer, and other experimental limitations.
While it's not typical for the actual yield to exceed the theoretical yield, there could be some cases where this might happen. One possible scenario is if there were errors in the measurements of reactants, and the recorded amounts were less than the actual amounts present in the reaction. Another potential scenario could involve impurities in the reactants that contribute to the mass measured but do not participate in the reaction.
In practice, chemists strive to maximize the actual yield and minimize sources of error to approach the theoretical yield as closely as possible. If the actual yield exceeds the theoretical yield, it's crucial to carefully analyze the experimental procedures and data to identify and understand any potential sources of error or miscalculation.
Under what circumstances might an actual yield surpass the theoretical yield?
In general, the actual yield of a chemical reaction is expected to be lower than the theoretical yield. This is because the theoretical yield assumes perfect conditions, which are rarely achieved in real-world experiments. However, there are a few specific circumstances under which an actual yield might surpass the theoretical yield:
1. Measurement errors:
- Mass of reactants: If the mass of a reactant is accidentally overestimated, the theoretical yield will be too low, and the actual yield could potentially appear to be higher. This can happen due to inaccurate weighing, contamination of the reactant, or absorption of moisture.
- Mass of product: If the mass of the product is accidentally overestimated due to impurities or incomplete drying, the actual yield will be artificially inflated.
2. Side reactions:
- In some cases, a side reaction can occur alongside the desired reaction, producing additional product. This additional product is not accounted for in the theoretical yield, so the actual yield could be higher. However, this scenario is typically not desirable as it dilutes the desired product and reduces its purity.
3. Absorption of reactants:
- In some reactions, the reactant can be adsorbed onto the surface of the reaction vessel or equipment used. This adsorbed reactant is not available for the reaction, leading to a lower actual yield. However, if the vessel or equipment is not cleaned properly before subsequent experiments, some of the adsorbed reactant could be released and participate in the reaction, leading to an apparent increase in the actual yield.
4. Incomplete product separation:
- If the product is not completely separated from the reaction mixture, the actual yield will be artificially inflated. This can happen due to inefficient separation techniques, incomplete drying of the product, or the presence of volatile impurities.
5. Catalytic reactions:
- In some catalytic reactions, the catalyst can promote the formation of products even in the absence of the ideal stoichiometric ratios of reactants. This can lead to an actual yield exceeding the theoretical yield based on the initial amount of reactants.
It's important to note that even in these circumstances, exceeding the theoretical yield is not guaranteed. The actual yield can still be lower than the theoretical yield due to other factors such as incomplete reactions, impurities, and losses during product isolation.
It's crucial to carefully analyze any experiment where the actual yield appears to exceed the theoretical yield to determine the cause of this discrepancy. Measurement errors and incomplete product separation should be considered first, followed by the possibility of side reactions or catalytic effects.