How to go from moles to liters?
Converting from moles to liters involves using the ideal gas law, specifically the relationship between moles (), pressure (), volume (), and the gas constant (). The ideal gas law is typically written as:
where:
- is the pressure of the gas,
- is the volume of the gas,
- is the number of moles of gas,
- is the ideal gas constant (0.0821 L atm / (mol K)),
- is the temperature of the gas in Kelvin.
Assuming constant temperature and pressure, you can rearrange the ideal gas law to solve for volume ():
Here's how to go from moles to liters using this formula:
Determine the Number of Moles ():
- This is the quantity of the substance given in moles.
Identify the Gas Constant ():
- The ideal gas constant is usually given as 0.0821 L atm / (mol K). Make sure to use consistent units.
Determine the Pressure ():
- If the pressure is not given, it is assumed to be constant. If the pressure changes, you need to know the pressure at the specific conditions you are considering.
Determine the Temperature ():
- Ensure the temperature is in Kelvin. If the temperature is given in Celsius, add 273.15 to convert it to Kelvin.
Apply the Formula:
- Plug the values of , , , and into the formula .
Perform the Calculation:
- Calculate the result to find the volume () in liters.
Here's an example:
Suppose you have 2 moles of a gas at a pressure of 1 atmosphere, a temperature of 300 Kelvin, and you want to find the volume.
So, 2 moles of the gas at the given conditions occupy a volume of 49.26 liters.
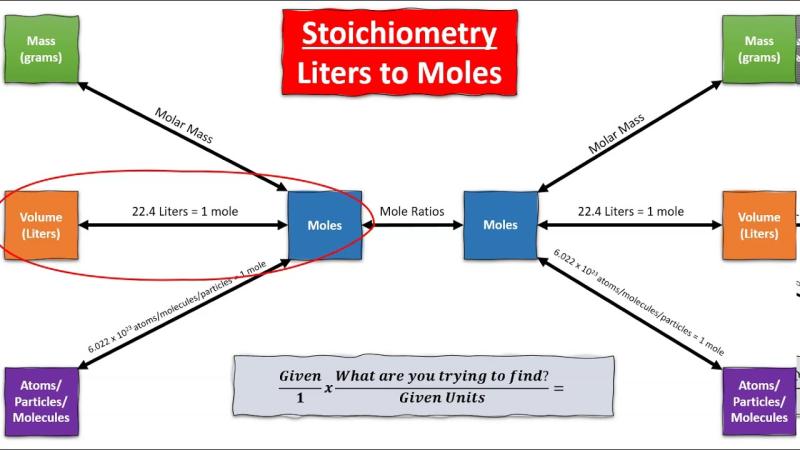
Chemical conversion: How to go from moles to liters?
In chemistry, converting between moles and liters is a fundamental skill for understanding and performing various calculations. Moles represent the amount of a substance in terms of the number of fundamental units, which can be atoms, molecules, or ions. Liters, on the other hand, represent the volume of a substance. The conversion between moles and liters is essential for preparing solutions, balancing chemical equations, and analyzing the concentration of solutions.
Explaining the conversion process from moles to liters in chemistry
The conversion between moles and liters involves the concept of molar volume, which is the volume occupied by one mole of a gas at standard temperature and pressure (STP). At STP, the molar volume of any gas is approximately 22.4 liters per mole (L/mol).
To convert moles to liters, you can use the following formula:
liters = moles × molar volume
For instance, if you have 2 moles of oxygen gas (O2) at STP, you can calculate the volume using the formula:
liters = 2 moles × 22.4 L/mol ≈ 44.8 liters
This indicates that 2 moles of oxygen gas at STP occupy a volume of approximately 44.8 liters.
Tips for students and scientists in performing accurate mole-to-liter conversions
Here are some tips for students and scientists in performing accurate mole-to-liter conversions:
Ensure you have the correct units: Moles are expressed in moles (mol), and liters are expressed in liters (L).
Use the molar volume correctly: The molar volume is typically used for gases at STP. For solids and liquids, the density or specific volume is used.
Be mindful of significant digits: Maintain the correct number of significant digits throughout the calculation to ensure accuracy.
Double-check your work: Always review your calculations to avoid errors.
Mole-to-liter conversions are crucial for various chemical applications. By following these steps and tips, students and scientists can perform mole-to-liter conversions accurately and confidently.