Can you divide grams by milliliters?
Yes, you can divide grams by milliliters, but the result will give you the density of the substance in grams per milliliter (g/mL). Density is a physical property that represents the mass of a substance per unit volume.
The formula for density is:
Density (g/mL) = Mass (grams) / Volume (milliliters)
So, if you have a certain mass of a substance in grams and you know its volume in milliliters, you can calculate its density by dividing the mass by the volume. This is a common calculation in chemistry and is used to characterize the physical properties of substances.
Converting Grams to Milliliters: Is It Possible?
Yes, it is possible to convert grams to milliliters, but it is not a straightforward conversion. Grams are a unit of mass, while milliliters are a unit of volume. In order to convert from grams to milliliters, you need to know the density of the substance you are converting.
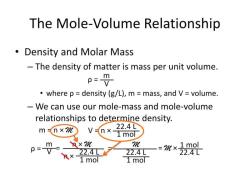
Density is defined as mass per unit volume. It is a measure of how much mass is contained in a given volume. The density of a substance is typically expressed in grams per cubic centimeter (g/cm³) or grams per milliliter (g/mL).
To convert grams to milliliters, you can use the following formula:
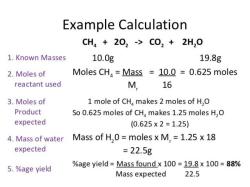
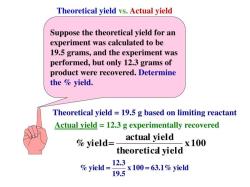
Volume (mL) = Mass (g) / Density (g/mL)
For example, if you want to convert 10 grams of water to milliliters, you would use the following calculation:
Volume (mL) = 10 grams / 1 g/mL = 10 mL
Exploring the Relationship Between Weight and Volume in Chemistry
The relationship between weight and volume in chemistry is complex and depends on the substance in question. In general, substances with a higher density will have a smaller volume than substances with a lower density. This is because density is a measure of how much mass is contained in a given volume.
For example, 10 grams of water will have a smaller volume than 10 grams of cotton. This is because water has a higher density than cotton.
When Mass Meets Volume: The Conversion Challenge
Converting from mass to volume can be challenging, especially when dealing with substances that have different densities. The key to converting from mass to volume is to know the density of the substance you are converting.
If you do not know the density of the substance you are converting, you can look it up in a chemistry reference book or online. Once you know the density, you can use the formula above to convert from grams to milliliters.
Here are some additional tips for converting from mass to volume:
- Make sure that the units you are using are consistent. For example, if you are converting grams to milliliters, you need to use the density of the substance in grams per milliliter.
- Be careful when converting from mass to volume for substances that are mixed. The density of a mixture will depend on the densities of the individual substances in the mixture.
- If you are unsure about how to convert from mass to volume, it is always best to consult with a chemist or other qualified professional.