What is the formula for molar concentration?
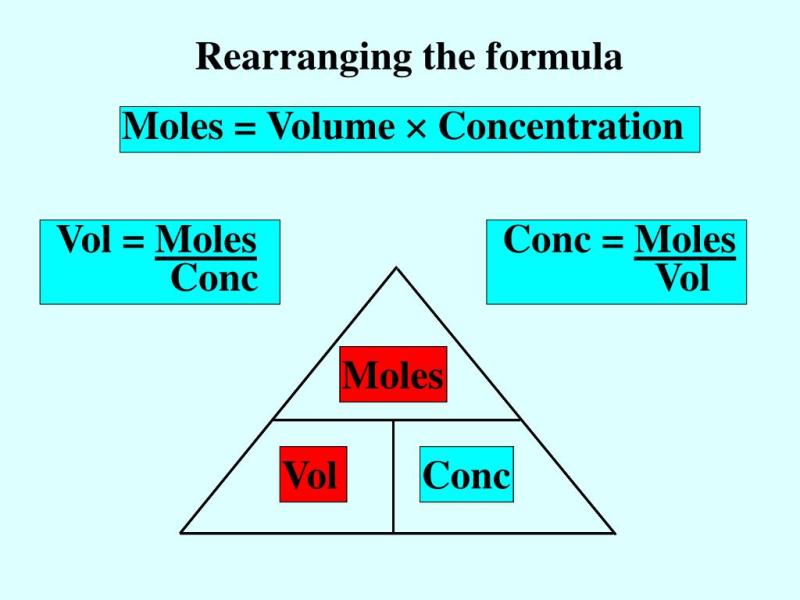
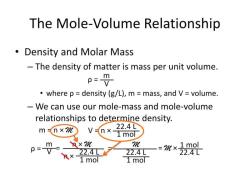
Molar concentration, also known as molarity, is a measure of the concentration of a solute in a solution. It is defined as the number of moles of solute per liter of solution. The formula for molar concentration (C) is:
In mathematical terms:
where:
- is the molar concentration in moles per liter (mol/L or M, which stands for molarity).
- is the number of moles of solute.
- is the volume of the solution in liters.
To use this formula, you need to know the number of moles of solute and the volume of the solution in liters. The result will give you the concentration of the solute in the solution.
For example, if you have 2 moles of solute in a solution with a volume of 1 liter, the molar concentration would be or 2 mol/L.
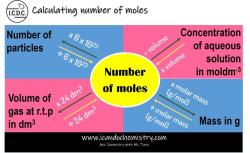
Molar concentration formula: What is the formula for molar concentration?
Molar concentration, also known as molarity, is a measure of the concentration of a solute in a solution. It is defined as the number of moles of solute per liter of solution. The molar concentration formula is:
M = n/V
where:
- M is the molar concentration in mol/L
- n is the number of moles of solute
- V is the volume of solution in liters
Introducing the formula for calculating molar concentration and its applications
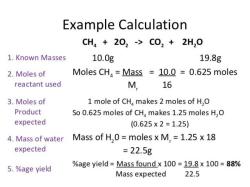
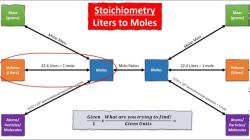
To calculate the molar concentration of a solution, you need to know the number of moles of solute and the volume of solution. The number of moles of solute can be calculated from its mass and molar mass. The molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol). The volume of solution is usually measured in liters (L).
Molar concentration is a useful unit of measurement for a variety of applications in chemistry, including:
- Preparing solutions: Molar concentration is used to prepare solutions with a specific concentration of solute.
- Chemical reactions: Molar concentration is used to stoichiometrically balance chemical equations.
- Analyzing solutions: Molar concentration is used to analyze solutions to determine the concentration of solute.
Tips for students and scientists in performing molar concentration calculations
Here are some tips for students and scientists in performing molar concentration calculations:
- Make sure you have the correct units: Molar concentration is expressed in mol/L, so make sure you have the number of moles of solute in moles and the volume of solution in liters.
- Use the correct molar mass: The molar mass of a substance can be found on the periodic table or in a reference book.
- Be careful with significant digits: When calculating molar concentration, make sure you carry the correct number of significant digits throughout the calculation.
- Double-check your work: Always double-check your work to make sure you have made no mistakes.
Molar concentration is a fundamental concept in chemistry and is essential for understanding chemical reactions and preparing solutions. By following these tips, you can perform molar concentration calculations accurately and confidently.