What is SN1 Prime reaction mechanism in organic chemistry?
I'm not aware of a specific reaction mechanism referred to as "SN1 Prime" in organic chemistry. However, the "SN1" reaction mechanism is a well-known and widely studied process in organic chemistry.
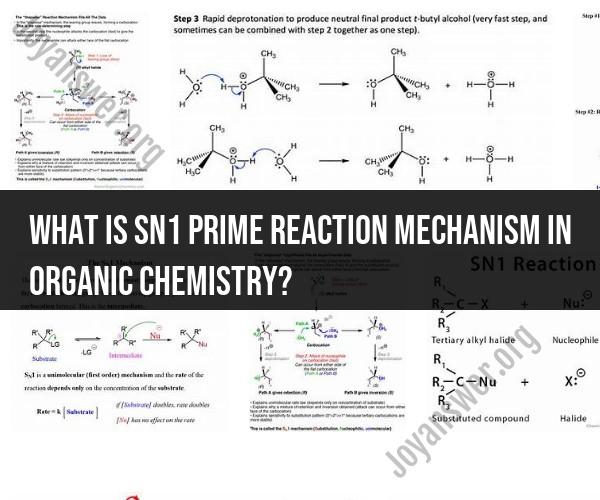
SN1 stands for "Substitution Nucleophilic Unimolecular." It's one of the two main mechanisms involved in nucleophilic substitution reactions, with the other being SN2 (Substitution Nucleophilic Bimolecular).
In an SN1 reaction, the key features include:
Unimolecular Reaction: The rate-determining step of the reaction involves the cleavage of a single chemical bond, typically resulting in the formation of a carbocation intermediate.
Stepwise Mechanism: The SN1 reaction proceeds through two distinct steps. In the first step, a leaving group departs from the substrate, forming a carbocation. In the second step, a nucleophile (a species with a pair of electrons) attacks the carbocation to complete the substitution.
Stereochemistry: The SN1 reaction often results in the formation of both retention and inversion of configuration at a chiral center when the reaction occurs at a stereocenter.
Solvent Effects: The choice of solvent can significantly impact the rate and outcome of SN1 reactions. Polar protic solvents (such as water or alcohol) are commonly used, as they stabilize the carbocation intermediate.
It's possible that the term "SN1 Prime" is used in specific contexts or by certain sources to describe a variation or an extension of the SN1 mechanism, but it's not a standard or widely recognized term in organic chemistry. If you have more specific information or context about "SN1 Prime," I'd be happy to provide more detailed information if it exists in the literature or in a particular field of study.
SN1 Prime Reaction Mechanism in Organic Chemistry: Insights
The SN1 prime reaction mechanism is a type of nucleophilic substitution reaction that occurs in organic chemistry. It is a unimolecular reaction, meaning that the rate-determining step involves the formation of a carbocation intermediate.
The SN1 prime reaction mechanism is similar to the SN1 reaction mechanism, but it differs in one key way. In the SN1 prime reaction mechanism, the nucleophile attacks the carbocation intermediate from the opposite side of the leaving group. This is because the nucleophile is attracted to the carbocation intermediate from the opposite side of the leaving group, where there is less steric hindrance.
The SN1 prime reaction mechanism is typically observed in tertiary alkyl halides, where the carbocation intermediate is highly stable. It is also observed in cases where the nucleophile is small and unhindered.
Here is an example of a SN1 prime reaction:
(CH3)3CCl + CH3OH → (CH3)3COH + CH3Cl
In this reaction, the tertiary alkyl halide (CH3)3CCl undergoes a SN1 prime reaction with methanol (CH3OH) to form the tertiary alcohol (CH3)3COH and methyl chloride (CH3Cl).
Organic Chemistry Mysteries: Demystifying SN1 Prime
The SN1 prime reaction mechanism is often overlooked in organic chemistry textbooks, but it is an important reaction mechanism to understand. The SN1 prime reaction mechanism can be observed in a variety of organic reactions, and it is important to be able to identify it when it occurs.
One of the challenges of understanding the SN1 prime reaction mechanism is that it is difficult to visualize. The carbocation intermediate is a short-lived species, and it is difficult to determine the exact orientation of the nucleophile when it attacks the carbocation intermediate.
However, there are a few things that can help you to understand the SN1 prime reaction mechanism. First, it is important to understand the concept of steric hindrance. Steric hindrance is the repulsion between non-bonded electron pairs and atoms. In the SN1 prime reaction mechanism, the nucleophile attacks the carbocation intermediate from the opposite side of the leaving group because there is less steric hindrance in this position.
Second, it is important to understand the concept of electronegativity. Electronegativity is the ability of an atom to attract electrons. In the SN1 prime reaction mechanism, the nucleophile is typically an atom or molecule with a high electronegativity. This is because the nucleophile is attracted to the positive charge on the carbocation intermediate.
Reaction Mechanism Spotlight: Understanding SN1 Prime
The SN1 prime reaction mechanism is a complex reaction mechanism, but it is an important one to understand. By understanding the SN1 prime reaction mechanism, you will be better equipped to solve organic chemistry problems and to understand the mechanisms of a variety of organic reactions.
Here are some additional tips for understanding the SN1 prime reaction mechanism:
- Practice drawing reaction mechanisms. The more you practice drawing reaction mechanisms, the better you will become at understanding them.
- Use reference materials. There are a number of reference materials available that can help you to understand the SN1 prime reaction mechanism. These materials include organic chemistry textbooks, online resources, and videos.
- Ask for help from a teacher or tutor. If you are struggling to understand the SN1 prime reaction mechanism, ask for help from a teacher or tutor. They will be able to provide you with additional explanations and resources.
With a little effort, you can master the SN1 prime reaction mechanism and improve your understanding of organic chemistry.