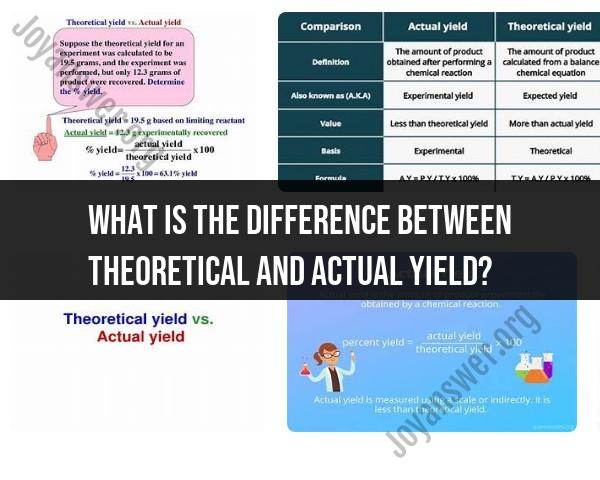
What is the difference between theoretical and actual yield?
Theoretical yield and actual yield are concepts used in chemistry to describe different aspects of the quantity of product produced in a chemical reaction.
Theoretical Yield:
Definition:
- The theoretical yield is the maximum amount of product that can be obtained from a chemical reaction, based on the stoichiometry of the reaction.
Calculation:
- It is calculated using balanced chemical equations and the stoichiometric coefficients of the reactants and products.
Ideal Conditions:
- Theoretical yield assumes that the reaction goes to completion, all reactants are fully converted into products, and there are no losses.
Theoretical Example:
- In the reaction A + B → C, if the stoichiometry indicates that one mole of A reacts with one mole of B to produce one mole of C, and you start with two moles of A and two moles of B, the theoretical yield of C is two moles.
Actual Yield:
Definition:
- The actual yield is the amount of product that is obtained in a real, experimental setting. It is the quantity of product that is collected and measured during or after a reaction.
Factors Affecting Actual Yield:
- Actual yield may be less than theoretical yield due to factors such as incomplete reactions, side reactions, impurities, experimental errors, or losses during product collection.
Calculation:
- It is determined through laboratory measurements. The actual yield is often expressed as a mass or volume of the product obtained.
Actual Example:
- Using the same example, if, due to experimental limitations, impurities, or incomplete reactions, you only obtain 1.8 moles of C from the reaction of two moles of A and two moles of B, the actual yield is 1.8 moles.
Yield Discrepancy:
Percentage Yield:
- The yield discrepancy or the efficiency of the reaction is often expressed as a percentage yield, calculated using the formula:
Interpretation:
- A percentage yield less than 100% indicates that the actual yield is less than the theoretical yield. Factors contributing to a lower actual yield should be considered and addressed.
Summary:
Theoretical yield is the calculated maximum amount of product based on stoichiometry and assumes ideal conditions.
Actual yield is the amount of product obtained through experimental measurements and reflects real-world conditions, which may result in a yield less than the theoretical.
Percentage yield expresses the efficiency of the reaction by comparing the actual and theoretical yields.
In summary, theoretical yield represents the ideal outcome of a chemical reaction, while actual yield reflects the practical result obtained in a laboratory or industrial setting, often affected by real-world constraints and limitations.
What distinguishes theoretical yield from actual yield in chemical reactions?
Both theoretical yield and actual yield are important concepts in chemical reactions, but they represent different aspects of the reaction process. Here's a breakdown of their distinctions:
Theoretical Yield:
- Definition: The maximum amount of product that can be formed from a given amount of reactants, assuming complete conversion and no loss.
- Calculation: Based on the balanced chemical equation and the limiting reactant. It's a stoichiometric calculation.
- Advantages: Provides a benchmark for the reaction's efficiency.
- Limitations: Doesn't account for real-world factors that can affect the reaction, leading to an overestimation of the actual yield.
Actual Yield:
- Definition: The actual amount of product obtained after the reaction is complete.
- Measurement: Determined experimentally by collecting and measuring the purified product.
- Advantages: Provides a realistic representation of the reaction's performance under specific conditions.
- Limitations: Can be influenced by various factors like incomplete reactions, side reactions, and losses during purification.
Factors Affecting Actual Yield:
- Incomplete reactions: Not all reactants might react fully due to various factors like temperature, reaction time, catalyst efficiency, and reactant purity.
- Side reactions: Unintended reactions can occur alongside the main reaction, consuming reactants and forming unwanted products.
- Losses during purification: Product isolation and purification processes can lead to product loss, reducing the actual yield.
- Heating effects: Exothermic reactions can generate unwanted heat, potentially leading to product decomposition or side reactions.
- Catalyst efficiency: Catalysts can accelerate the reaction, but their activity can decrease over time, affecting the actual yield.
Percent Yield:
- Calculation: (Actual yield / Theoretical yield) x 100%
- Interpretation: Indicates the efficiency of the reaction in converting reactants to products. A higher percentage yield indicates a more efficient process.
Understanding the differences between theoretical and actual yield is crucial for evaluating the effectiveness of chemical reactions and optimizing them for better performance. By analyzing the various factors influencing the actual yield, scientists can improve reaction conditions, minimize losses, and ultimately maximize product formation.