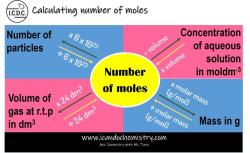
How do you calculate the molarity of a solution?
To calculate the molarity (M) of a solution, you need to know the amount of solute (usually measured in moles) and the volume of the solution (usually measured in liters). The formula for calculating molarity is:
Here's a step-by-step guide on how to calculate the molarity of a solution:
Step 1: Gather Information
You will need to know the following information:
The amount of solute (measured in moles): This is the quantity of the substance that is dissolved in the solution. You can often find this information in the problem statement or through experimental data.
The volume of the solution (measured in liters): This is the total volume of the solution, including both the solvent and the solute. Ensure that the volume is measured in liters, and if not, convert it to liters.
Step 2: Plug the Values into the Formula
Use the formula for molarity and plug in the values you have gathered:
Step 3: Perform the Calculation
Divide the moles of solute by the volume of the solution in liters to calculate the molarity:
Step 4: Express the Result
Express the molarity in units of moles per liter (mol/L) or Molar (M). The Molar is a common unit for molarity.
Example: Calculating Molarity
Let's work through an example:
Suppose you have 0.5 moles of table salt (NaCl) dissolved in 2 liters of water to make a solution. Calculate the molarity (M) of the solution.
Gather Information:
- Moles of NaCl = 0.5 moles
- Volume of solution = 2 liters
Plug Values into the Formula:
Perform the Calculation:
Express the Result:
The molarity of the solution is 0.25 M (0.25 moles per liter).
So, the solution has a molarity of 0.25 M, which means there are 0.25 moles of NaCl in each liter of the solution.