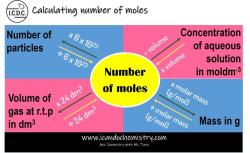
What is KC and KP?
In the context of chemical equilibrium, KC and KP are two different ways to express the equilibrium constant for a chemical reaction. They represent the relationship between the concentrations (in the case of KC) or the partial pressures (in the case of KP) of products and reactants at equilibrium.
KC (Equilibrium Constant in Terms of Concentrations):
KC is used when the concentrations of reactants and products are expressed in molarity (M), which is a measure of the number of moles of a substance per unit volume of the solution.
The equilibrium constant expression (KC) for a generic chemical reaction is given by:
KC = [C]^c [D]^d / [A]^a [B]^b
Where:
- [A], [B], [C], and [D] are the molar concentrations of the reactants A and B and the products C and D, respectively.
- "a," "b," "c," and "d" are the coefficients from the balanced chemical equation for the reaction.
KC is used for reactions taking place in solution, and it quantifies the position of equilibrium based on the concentrations of the substances involved.
KP (Equilibrium Constant in Terms of Partial Pressures):
KP is used when the concentrations of reactants and products are expressed in terms of partial pressures, especially for gas-phase reactions.
The equilibrium constant expression (KP) for a generic chemical reaction is given by:
KP = (PC)^c (PD)^d / (PA)^a (PB)^b
Where:
- PA, PB, PC, and PD are the partial pressures of the gases A, B, C, and D, respectively.
- "a," "b," "c," and "d" are the coefficients from the balanced chemical equation for the reaction.
KP is particularly useful when dealing with gas-phase reactions because it takes into account the fact that gas pressures depend on the number of moles of gas as well as temperature and the ideal gas constant.
The values of KC and KP are determined experimentally for a given chemical reaction at a specific temperature. These equilibrium constants provide information about the extent to which a reaction proceeds toward products (larger KC or KP values indicate more products at equilibrium) or toward reactants (smaller KC or KP values indicate more reactants at equilibrium). The specific value of KC or KP also provides insights into the reaction's equilibrium position, but it's important to note that the numerical values of these constants can vary significantly depending on the reaction and conditions.
KC and KP are equilibrium constants that are used to describe the state of a chemical reaction at equilibrium. KC is the equilibrium constant expressed in terms of concentrations, while KP is the equilibrium constant expressed in terms of partial pressures.
KC is calculated using the following equation:
KC = [products] / [reactants]
where [products] and [reactants] are the concentrations of the products and reactants at equilibrium, respectively.
KP is calculated using the following equation:
KP = (Pproducts) / (Preactants)
where (Pproducts) and (Preactants) are the partial pressures of the products and reactants at equilibrium, respectively.
The relationship between KC and KP is given by the following equation:
KP = KC(RT)^Δn
where R is the ideal gas constant, T is the temperature in Kelvin, and Δn is the change in the number of moles of gas from reactants to products.
KC and KP are useful for predicting the direction of a chemical reaction and the equilibrium concentrations of the products and reactants. For example, if KC is greater than 1, then the reaction will proceed to form products at equilibrium. If KC is less than 1, then the reaction will proceed to form reactants at equilibrium.
Here is an example of how to use KC to predict the direction of a chemical reaction:
H2(g) + I2(g) ⇌ 2HI(g)
The equilibrium constant for this reaction is KC = 50 at 25°C. If we start with equal concentrations of H2 and I2, then the reaction will proceed to form products at equilibrium because KC is greater than 1.
KC and KP are important concepts in chemistry and are used in a variety of applications, such as chemical engineering, analytical chemistry, and environmental chemistry.