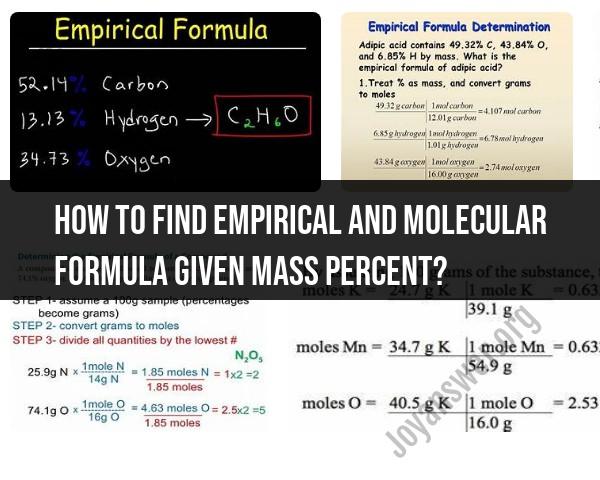
How to find empirical and molecular formula given mass percent?
To find the empirical and molecular formulas from mass percent data, you need to follow a step-by-step process. Here's a guide on how to do it:
1. Understand the Definitions:
- The empirical formula represents the simplest, whole-number ratio of elements in a compound.
- The molecular formula represents the actual number of atoms of each element in a molecule.
2. Convert Mass Percent to Grams:
- Assume you have a 100g sample of the compound. This makes mass percent equivalent to grams, simplifying calculations.
3. Determine Moles of Each Element:
- Convert the grams of each element to moles using the molar mass of each element (found on the periodic table).
4. Find the Simplest Ratio:
- Determine the simplest whole-number ratio of moles of each element by dividing each value by the smallest number of moles.
5. Write the Empirical Formula:
- Use the ratios obtained to write the empirical formula. If the ratios are very close to whole numbers, round them to the nearest whole number.
6. Calculate the Molecular Formula:
- If the molecular formula is different from the empirical formula, find the molecular formula using additional information, such as the molecular mass.
- is the factor by which the empirical formula must be multiplied to get the molecular formula.
Example:
Suppose you have a compound with the following mass percent composition:
- Element X: 40.0%
- Element Y: 60.0%
- Assume a 100g sample, so you have 40.0g of X and 60.0g of Y.
- Convert grams to moles:
- Find the mole ratio: If moles of X = 2 and moles of Y = 3, the ratio is 2:3.
- Write the empirical formula: .
- If additional information (e.g., molecular mass) is given, calculate the molecular formula using the given formula mass and the empirical formula mass.
This is a general approach, and the specific steps may vary based on the information provided in the problem. Always check for whole-number ratios and round appropriately.
Unlocking chemical formulas: A guide to finding empirical and molecular formulas from mass percent
Chemical formulas are used to represent the composition of compounds. The empirical formula gives the simplest whole-number ratio of atoms of each element in a compound, while the molecular formula gives the actual number of atoms of each element in a molecule of the compound.
To find the empirical and molecular formulas of a compound from mass percent data, you can follow these steps:
- Convert the mass percent data to moles. To do this, divide the mass percent of each element by its atomic mass and then multiply by 100.
- Find the mole ratio of the elements. Divide the number of moles of each element by the smallest number of moles. This will give you the simplest whole-number mole ratio of the elements in the compound.
- Write the empirical formula. The empirical formula is made up of the chemical symbols of the elements in the compound, with the number of atoms of each element represented by a subscript. For example, the empirical formula for water is H2O, which means that there are 2 hydrogen atoms and 1 oxygen atom in a molecule of water.
- Find the molecular formula. To find the molecular formula, you need to know the molar mass of the compound. Once you know the molar mass, you can divide it by the molar mass of the empirical formula to get a factor. Multiply the subscripts in the empirical formula by this factor to get the molecular formula.
Step-by-step calculations for determining empirical and molecular formulas using mass percent data
Here is an example of how to determine the empirical and molecular formulas of a compound from mass percent data:
Mass percent data:
- Carbon: 40.0%
- Hydrogen: 6.7%
- Oxygen: 53.3%
Step 1: Convert the mass percent data to moles.
C: 40.0 g / 12.011 g/mol = 3.33 mol
H: 6.7 g / 1.008 g/mol = 6.63 mol
O: 53.3 g / 15.999 g/mol = 3.33 mol
Step 2: Find the mole ratio of the elements.
C: 3.33 mol / 3.33 mol = 1
H: 6.63 mol / 3.33 mol = 2
O: 3.33 mol / 3.33 mol = 1
Step 3: Write the empirical formula.
CH2O
Step 4: Find the molecular formula.
To find the molecular formula, we need to know the molar mass of the compound. The molar mass of the empirical formula, CH2O, is 30.03 g/mol.
We can divide the molar mass of the compound by the molar mass of the empirical formula to get a factor:
30.03 g/mol / 30.03 g/mol = 1
Multiplying the subscripts in the empirical formula by this factor gives us the molecular formula:
CH2O
Therefore, the empirical and molecular formulas of the compound are both CH2O.
Tips for solving complex chemical problems involving empirical and molecular formulas
When solving complex chemical problems involving empirical and molecular formulas, it is important to keep the following tips in mind:
- Make sure that you understand the basic concepts of empirical and molecular formulas. This includes understanding the difference between the two types of formulas and how to calculate them.
- Be careful with conversions. When converting from mass percent to moles and vice versa, make sure that you are using the correct atomic masses.
- Check your work. After you have calculated the empirical and molecular formulas, be sure to check your work to make sure that you made no errors.
Here are some additional tips for solving complex chemical problems involving empirical and molecular formulas:
- Use diagrams and charts. Diagrams and charts can be helpful for visualizing the chemical relationships involved in the problem.
- Break down the problem into smaller parts. This can make the problem seem less overwhelming and easier to solve.
- Look for patterns. Many chemical problems involve repeating patterns. Once you identify a pattern, you can use it to solve the problem more easily.
- Don't be afraid to ask for help. If you are stuck on a problem, ask your teacher or a classmate for help.
By following these tips, you can improve your ability to solve complex chemical problems involving empirical and molecular formulas.