Is concentration and molarity the same thing?
"Concentration" and "molarity" are related terms but refer to different concepts in the context of solutions and chemistry.
Concentration:
Concentration is a broad term that describes the amount of a substance (the solute) present in a given volume or mass of a solution. There are various ways to express concentration, and the units depend on the context:
Mass Concentration (Mass Percent): It is the mass of the solute divided by the total mass of the solution, multiplied by 100 to get a percentage.
Volume Concentration (Volume Percent): It is the volume of the solute divided by the total volume of the solution, multiplied by 100.
Molarity:
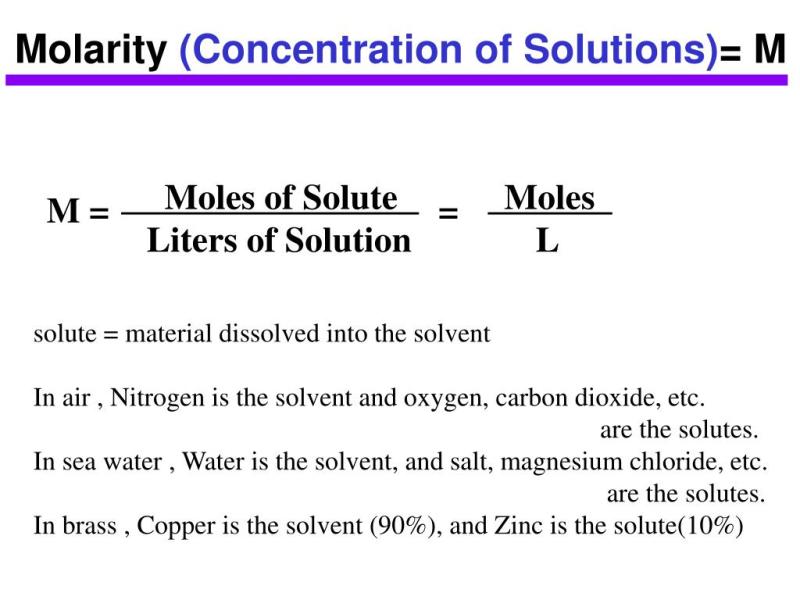
Molarity (M) is a specific type of concentration that expresses the number of moles of solute per liter of solution. It is a widely used unit of concentration, especially in chemical reactions.
In summary, while "concentration" is a general term encompassing various ways to express the amount of solute in a solution, "molarity" specifically refers to the concentration expressed in moles per liter. Other concentration measures, such as mass percent or volume percent, convey information about the relative amount of solute in a solution but may not directly involve the concept of moles.
When working with chemical reactions and laboratory experiments, molarity is often the preferred unit of concentration because it directly relates to the stoichiometry of reactions. However, the choice of concentration unit depends on the specific needs of the analysis or experiment.
Chemistry terminology: Is concentration the same as molarity?
No, concentration and molarity are not the same. Concentration is a general term that refers to the amount of a substance dissolved in another substance. Molarity is a specific type of concentration that is measured in moles per liter (mol/L).
Clarifying the distinctions between concentration and molarity in chemistry
Concentration can be expressed in a variety of ways, including:
- Mass percent (%)
- Volume percent (%)
- Parts per million (ppm)
- Parts per billion (ppb)
- Mole fraction
- Molarity
Molarity is a measure of the concentration of a solution in terms of the number of moles of solute per liter of solution. It is calculated using the following formula:
Molarity = moles of solute / liters of solution
For example, a 1 M solution of sodium chloride (NaCl) contains 1 mole of NaCl per liter of solution.
Tips for students and researchers in understanding and applying these concepts in chemical analysis
To understand and apply the concepts of concentration and molarity in chemical analysis, it is important to keep the following in mind:
- Concentration is a measure of the amount of a substance dissolved in another substance.
- Molarity is a specific type of concentration that is measured in moles per liter (mol/L).
- Concentration can be expressed in a variety of ways, but molarity is the most common unit of concentration used in chemistry.
- Molarity is useful for calculating the amount of solute needed to prepare a solution of a specific concentration, or for calculating the concentration of a solution after a chemical reaction has occurred.
Here are some specific examples of how concentration and molarity can be used in chemical analysis:
- To prepare a 1 M solution of NaCl, you would dissolve 58.44 grams of NaCl in 1 liter of water.
- To calculate the concentration of a solution of NaOH after it has reacted with HCl, you would use the following equation:
Molarity of NaOH = moles of NaOH / liters of solution
The moles of NaOH can be calculated from the volume and concentration of the HCl solution used in the reaction.