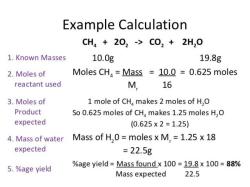
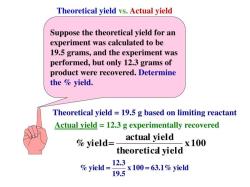
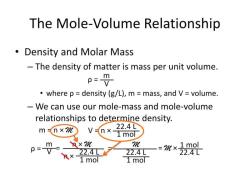
What are some common household acids and bases?
Common household acids and bases are substances that many people encounter in their daily lives. Here are some everyday examples of both:
Common Household Acids:
Vinegar: Vinegar, which is a weak acetic acid solution, is used in cooking and cleaning. It imparts a sour taste to foods and is often used as a condiment or salad dressing.
Citrus Fruits: Citrus fruits like lemons and oranges contain citric acid, which gives them their tart flavor. They are used in cooking and beverages.
Tomatoes: Tomatoes contain citric acid, as well as other acids, which give them their characteristic tangy taste.
Yogurt: Yogurt contains lactic acid, which contributes to its slightly sour taste.
Battery Acid: In household batteries, sulfuric acid is used as an electrolyte. It is highly corrosive and should be handled with extreme care.
Common Household Bases:
Baking Soda: Baking soda, or sodium bicarbonate, is a common base used in baking and for various cleaning purposes. It can help neutralize acids.
Ammonia: Ammonia is often used as a cleaning agent. It is an alkaline substance and can help remove stains and odors.
Antacids: Many over-the-counter antacids, like Tums, Maalox, or Rolaids, contain bases such as calcium carbonate or magnesium hydroxide. They are used to neutralize stomach acid and provide relief from heartburn and indigestion.
Soap: Soap is typically slightly basic. It helps to emulsify and remove oils and dirt from surfaces when used for cleaning.
Oven Cleaner: Oven cleaners often contain strong alkaline substances, such as sodium hydroxide (lye), to help break down and remove burnt-on food residues.
It's important to note that while some of these household acids and bases are safe for common use, others can be corrosive or harmful. Always follow safety guidelines and use them as intended, and avoid mixing household chemicals, as some combinations can produce hazardous reactions.
Common Household Acids and Bases: What You Should Know
Acids and bases are two important types of chemicals. Acids are substances that donate hydrogen ions (H+) in water, while bases are substances that accept hydrogen ions (H+) in water.
Common household acids include:
- Vinegar (acetic acid)
- Lemon juice (citric acid)
- Battery acid (sulfuric acid)
- Oxalic acid (found in cleaning products and bleach)
- Hydrochloric acid (found in toilet bowl cleaner and rust remover)
Common household bases include:
- Baking soda (sodium bicarbonate)
- Ammonia (found in window cleaner and glass cleaner)
- Lye (sodium hydroxide)
- Washing soda (sodium carbonate)
- Milk of magnesia (magnesium hydroxide)
Everyday Uses and Applications of Household Acids
Acids are used in a variety of household products and applications, including:
- Cleaning products: Acids can be used to remove dirt, grime, and stains. For example, vinegar is often used to clean windows and floors.
- Food preparation: Acids can be used to add flavor to food, such as lemon juice on fish or vinegar on salad.
- Gardening: Acids can be used to adjust the pH of soil and to kill weeds. For example, oxalic acid is often used to remove rust from concrete and other surfaces.
Recognizing Common Bases Found in Household Products
Bases are also used in a variety of household products and applications, including:
- Cleaning products: Bases can be used to remove grease and oil stains. For example, ammonia is often used to clean windows and mirrors.
- Personal care products: Bases are used in a variety of personal care products, such as toothpaste and shampoo.
- Food preparation: Bases are used in some food preparation processes, such as baking soda is used to make bread rise.
Handling and Safety Considerations with Household Acids and Bases
Acids and bases can be dangerous if not handled properly. It is important to follow the safety precautions on the product label when using acids and bases.
Here are some general safety tips for handling acids and bases:
- Always wear gloves and eye protection when handling acids and bases.
- Work in a well-ventilated area.
- Avoid contact with skin and eyes.
- If you do get acid or base on your skin, rinse it off immediately with water.
- If you get acid or base in your eyes, flush your eyes with water for 15 minutes and seek medical attention immediately.
Household Acid-Base Reactions and Their Impact
Acids and bases can react with each other to form neutral salts and water. This is known as an acid-base reaction.
Acid-base reactions can occur in the household when two different acids or bases are mixed together. For example, if you mix vinegar (acetic acid) and baking soda (sodium bicarbonate), you will get a chemical reaction that produces carbon dioxide gas and sodium acetate.
Acid-base reactions can also occur when acids or bases react with other household products. For example, if you mix bleach (sodium hypochlorite) with ammonia (ammonium hydroxide), you will get a chemical reaction that produces toxic chlorine gas.
It is important to be aware of the potential for acid-base reactions when using household acids and bases. If you are unsure whether two products will react safely, it is best to err on the side of caution and avoid mixing them.
Overall, acids and bases are important types of chemicals that have a variety of uses in the household. However, it is important to handle and use acids and bases safely to avoid accidents.