How to calculate molarity from molecular weight?
To calculate the molarity (M) of a solution from its molecular weight and mass, you can use the following formula:
Here's how to calculate molarity from molecular weight and mass:
Step 1: Determine the Mass of the Solute
- Measure or determine the mass of the solute in grams (g). The solute is the substance you are dissolving in the solvent to make the solution.
Step 2: Determine the Molecular Weight (Molar Mass) of the Solute2. Find the molecular weight (also known as molar mass) of the solute. The molecular weight is typically expressed in grams per mole (g/mol). You can find this value on the periodic table or by adding up the atomic masses of all the atoms in the chemical formula of the solute.
Step 3: Use the Formula to Calculate Molarity3. Plug the values from steps 1 and 2 into the formula for molarity:
By performing this calculation, you'll determine the molarity of the solution. Molarity represents the number of moles of solute in one liter of solution (mol/L). If you want the result in a different volume unit, you can adjust accordingly.
Example: Let's say you have 5 grams of sodium chloride (NaCl) and want to calculate the molarity of the NaCl solution.
Solution:
- Determine the mass of the solute (NaCl): 5 grams.
- Find the molecular weight (molar mass) of NaCl:
- Sodium (Na): 22.99 g/mol
- Chlorine (Cl): 35.45 g/mol
- Molecular weight of NaCl: g/mol.
- Use the formula for molarity:
So, the molarity of the NaCl solution is approximately 0.0856 M. This means there are approximately 0.0856 moles of NaCl in 1 liter of the solution.
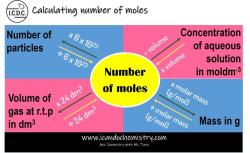
The Road to Molarity: Calculating from Molecular Weight
Molarity is a measure of the concentration of a solution. It is defined as the number of moles of solute per liter of solution. The mole is a unit of measurement that is used to count the number of particles in a substance. It is defined as the amount of substance that contains the same number of particles as there are atoms in 12 grams of carbon-12.
Molecular weight is the mass of one mole of a substance. It is expressed in grams per mole (g/mol).
To calculate molarity from molecular weight, you need to know the following:
- The mass of the solute (in grams)
- The volume of the solution (in liters)
- The molecular weight of the solute (in g/mol)
Once you have this information, you can use the following formula to calculate molarity:
Molarity = Mass of solute / (Volume of solution * Molecular weight of solute)
Understanding Molarity and Its Connection to Molecular Weight
Molarity and molecular weight are two closely related concepts. Molarity is a measure of the concentration of a solution, while molecular weight is a measure of the mass of one mole of a substance.
The connection between molarity and molecular weight is that molarity is calculated by dividing the mass of the solute by the volume of the solution and the molecular weight of the solute. This means that molarity is inversely proportional to molecular weight. In other words, the higher the molecular weight of a substance, the lower its molarity will be for a given mass of solute in a given volume of solution.
Using Molecular Weight for Molarity Calculations
Molecular weight can be used for molarity calculations in a number of ways. For example, it can be used to calculate the volume of solution needed to prepare a solution of a certain molarity. It can also be used to calculate the mass of solute needed to prepare a solution of a certain molarity.
Here is an example of how to use molecular weight for a molarity calculation:
Problem: Calculate the volume of a 1.00 M solution of sodium chloride (NaCl) that contains 10.0 grams of NaCl.
Solution:
- Calculate the molecular weight of NaCl:
Molecular weight of NaCl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
- Use the following formula to calculate the volume of the solution:
Volume of solution = Mass of solute / (Molarity of solution * Molecular weight of solute)
Volume of solution = 10.0 grams / (1.00 M * 58.44 g/mol) = 0.170 liters
Therefore, the volume of a 1.00 M solution of NaCl that contains 10.0 grams of NaCl is 0.170 liters.
Conclusion
Molecular weight is a useful concept for molarity calculations. It can be used to calculate the volume of solution needed to prepare a solution of a certain molarity, or to calculate the mass of solute needed to prepare a solution of a certain molarity.