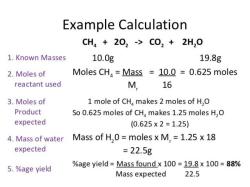
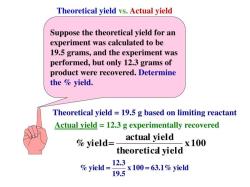
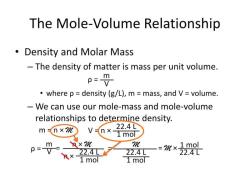
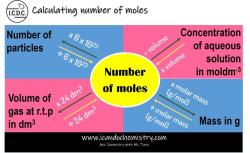
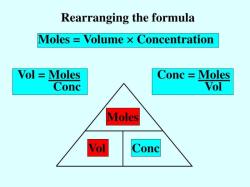
What is the Sir effect in organic chemistry?
The term "SIR effect" in organic chemistry usually refers to the Steric Inhibition of Resonance effect. The SIR effect is a concept that arises in the study of organic molecules and their reactivity. It is a steric factor that influences the resonance of electrons in a molecule, affecting its chemical properties.
In resonance, electrons in a molecule can move or delocalize, creating multiple resonance structures. The SIR effect occurs when steric hindrance, caused by bulky groups or substituents, restricts or inhibits the movement of electrons. This inhibition of electron movement can have several consequences in organic chemistry:
Reduced Resonance: The SIR effect can reduce the extent to which electrons can be delocalized through resonance. This can lead to a decrease in the stability of resonance structures.
Selective Reactivity: Molecules with the SIR effect may exhibit selective reactivity, as certain reactions may be hindered due to steric hindrance, while other reactions may be favored.
Steric Hindrance: The presence of bulky groups can hinder the approach of reagents or other molecules in a reaction, influencing the outcome of reactions, especially in situations involving electrophilic or nucleophilic attacks.
Product Selectivity: In some reactions, the SIR effect can lead to the preferential formation of one product over another due to steric factors that inhibit the formation of alternative products.
The SIR effect is often discussed in the context of organic chemistry, particularly in reaction mechanisms and reactivity studies. Understanding the impact of steric hindrance and the SIR effect is crucial for predicting and explaining the behavior of organic compounds in various chemical reactions. It is one of several factors, along with electronic effects, that influence the reactivity and behavior of organic molecules.
The "Sir Effect" in Organic Chemistry: A Phenomenon Explored
The "Sir Effect", also known as steric inhibition of resonance, is an organic chemistry phenomenon that occurs when a bulky substituent group is present in a position that prevents the resonance effect from taking place. This is because the bulky substituent group prevents the pi electrons from delocalizing, which reduces the stability of the molecule.
The Sir Effect is most commonly observed in aromatic compounds, where the bulky substituent group is present in the ortho position relative to the aromatic ring. For example, in the molecule ortho-methylnitrobenzene, the methyl group prevents the resonance effect between the nitro group and the aromatic ring. This reduces the stability of the molecule and makes it more reactive than if the methyl group were not present.
The Sir Effect can also be observed in other types of organic compounds, such as alkenes and alkynes. For example, in the molecule 2-methyl-2-butene, the methyl group prevents the resonance effect between the two double bonds. This reduces the stability of the molecule and makes it more reactive than if the methyl group were not present.
The Sir Effect is an important concept to understand in organic chemistry, as it can affect the stability and reactivity of molecules. It is also important to be aware of the Sir Effect when designing organic reactions, as it can affect the yield and selectivity of the reaction.
Organic Chemistry Wonders: Unraveling the "Sir Effect"
The Sir Effect is a fascinating phenomenon that reveals the complex and intricate nature of organic chemistry. It is a reminder that even small changes in the structure of a molecule can have a significant impact on its properties.
The Sir Effect is also a testament to the power of resonance. Resonance is a fundamental concept in organic chemistry that explains the stability and reactivity of many different types of molecules. The Sir Effect shows us that resonance is not always straightforward, and that it can be affected by the steric hindrance of bulky substituent groups.
The Sir Effect is also a reminder of the importance of understanding the three-dimensional structure of molecules. The Sir Effect can only occur when a bulky substituent group is present in a position that prevents the resonance effect from taking place. This means that it is important to understand the three-dimensional structure of a molecule in order to predict whether or not the Sir Effect will occur.
Chemical Enigma: Delving into the "Sir Effect"
The Sir Effect is a chemical enigma that continues to fascinate and puzzle researchers. There is still much that we do not know about how the Sir Effect works and how it affects the properties of molecules.
One of the challenges of studying the Sir Effect is that it is difficult to measure the resonance energy of a molecule directly. However, there are a number of indirect methods that can be used to estimate the resonance energy of a molecule.
Researchers are also using computational chemistry to study the Sir Effect. Computational chemistry allows researchers to simulate the behavior of molecules and to predict their properties. This information can be used to better understand how the Sir Effect works and how it affects the properties of molecules.
The Sir Effect is an important and fascinating phenomenon in organic chemistry. By understanding the Sir Effect, we can better understand the stability and reactivity of molecules. This knowledge can be used to design new organic reactions and to develop new materials.