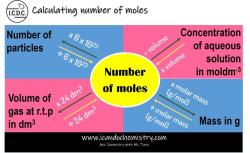
What is the mass of 18g of H2O in molecules?
To determine the number of molecules in 18 grams of water (H2O), you need to use Avogadro's number and the molar mass of water.
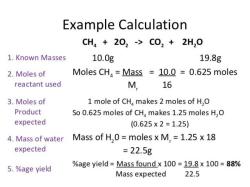
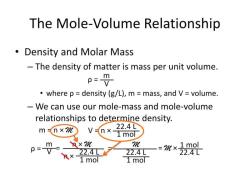
First, find the molar mass of water (H2O):
- The molecular formula of water (H2O) consists of 2 hydrogen (H) atoms and 1 oxygen (O) atom.
- The atomic mass of hydrogen (H) is approximately 1 atomic mass unit (amu), and the atomic mass of oxygen (O) is approximately 16 amu.
- So, the molar mass of water (H2O) is 2(1 amu for hydrogen) + 16 amu for oxygen = 18 grams per mole.
Now, you know that 1 mole of water (H2O) weighs 18 grams.
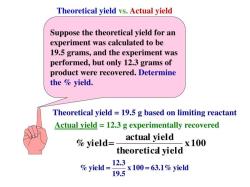
To find the number of molecules in 18 grams of water, you can use Avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. This number represents the number of molecules (or atoms) in one mole of any substance.
So, in 18 grams of water (H2O), you have:
- 1 mole of water, which contains approximately 6.022 x 10^23 molecules.
Therefore, in 18 grams of water, you have approximately 6.022 x 10^23 water molecules.