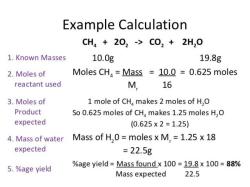
Which structures are used to protect amino groups in peptide synthesis?
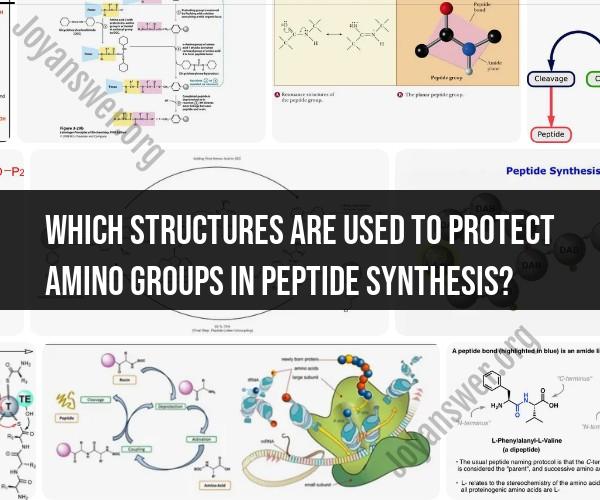
In peptide synthesis, amino group protection is a common practice to prevent unwanted reactions with amino groups at specific positions in the growing peptide chain. Several protecting groups, also known as "amino group protecting reagents," are used for this purpose. The choice of protecting group depends on the specific amino acid and its position in the peptide sequence. Here are some commonly used protecting groups in peptide synthesis:
Boc (tert-butyloxycarbonyl): The Boc group is a widely used protecting group. It is stable under a variety of conditions and can be removed using acid, such as trifluoroacetic acid (TFA). Boc protection is commonly used for the N-terminal amino group.
Fmoc (9-fluorenylmethyloxycarbonyl): The Fmoc group is a popular protecting group for the N-terminal amino group in solid-phase peptide synthesis (SPPS). It can be removed using piperidine, which makes it suitable for stepwise solid-phase synthesis.
t-Bu (tert-butyl): The t-Bu group is used to protect the side chains of amino acids, typically the hydroxyl groups of serine and threonine. It can be removed using acid, often in combination with other protecting group removal steps.
Cbz (benzyloxycarbonyl): The Cbz group is used to protect the side chains of amino acids containing amines, such as lysine and ornithine. It can be removed with hydrogenation or by treatment with acid.
Trt (trityl): The Trt group is often used to protect side-chain amines, such as the epsilon amino group of lysine, and can be removed with acid.
Alloc (allyloxycarbonyl): The Alloc group is used to protect side-chain amines and can be removed selectively using Pd(PPh3)4 and acid, making it suitable for orthogonal deprotection strategies.
Mtr (4-methoxytrityl): The Mtr group is employed for the protection of side-chain amines and can be removed selectively using mild acid conditions.
t-Butyl ester (t-Bu ester): In addition to protecting side-chain amino groups, the t-Bu ester is used to protect the carboxyl groups of aspartic acid and glutamic acid.
Alloc for Asp/Glu (allyloxycarbonyl for Asp/Glu): This protecting group is used to selectively protect the side-chain carboxyl groups of aspartic acid and glutamic acid.
TFA (trifluoroacetic acid)-labile protecting groups: Some protecting groups are designed to be removed using TFA, such as the TFA-labile Boc and Cbz groups.
The choice of protecting group depends on the specific synthetic strategy, the chemistry used, and the desired deprotection conditions. Protection and deprotection steps are critical in peptide synthesis to ensure the correct sequence of amino acids is obtained while avoiding undesired reactions. Additionally, orthogonal protecting groups are used to protect multiple functional groups in the same peptide molecule, allowing selective deprotection of specific groups while leaving others intact.
Protective structures and reagents for amino groups in peptide synthesis
There are many different protective groups and reagents that can be used to protect amino groups in peptide synthesis. Some of the most common include:
- Boc (tert-butoxycarbonyl): Boc is a base-labile protecting group that is often used to protect the amino group of the N-terminal amino acid in a peptide.
- Fmoc (9-fluorenylmethoxycarbonyl): Fmoc is an acid-labile protecting group that is often used to protect the amino groups of side chains in a peptide.
- Trt (trityl): Trt is an acid-labile protecting group that is often used to protect the amino group of the arginine side chain.
- Cbz (benzyloxycarbonyl): Cbz is a hydrogenolysis-labile protecting group that is often used to protect the amino group of the glutamic acid side chain.
Common methods to safeguard amino groups during peptide assembly
There are two common methods to safeguard amino groups during peptide assembly:
- Temporary protection: Temporary protection involves using a protecting group that can be easily removed after the peptide has been synthesized. This is the most common method of amino group protection in peptide synthesis.
- Permanent protection: Permanent protection involves using a protecting group that cannot be removed after the peptide has been synthesized. This method is less common, but it can be useful in some cases, such as when the peptide is to be used in a biological system where the amino group needs to be masked.
Challenges and considerations in amino group protection in chemistry
There are a number of challenges and considerations associated with amino group protection in chemistry. Some of the most important include:
- Compatibility: The protecting group must be compatible with the other reagents and conditions used in peptide synthesis.
- Selectivity: The protecting group should be selective for the amino group that needs to be protected.
- De-protection: The protecting group should be able to be removed easily and efficiently after the peptide has been synthesized.
- Cost: The protecting group should be cost-effective.
De-protection strategies to reveal amino groups in synthesized peptides
The specific de-protection strategy used depends on the type of protecting group that has been used. For example, Boc protecting groups can be removed with a base, such as trifluoroacetic acid (TFA), while Fmoc protecting groups can be removed with an acid, such as piperidine.
Advances in amino group protection techniques for peptide chemistry
There has been significant progress in the development of new and improved amino group protection techniques for peptide chemistry in recent years. Some of the most notable advances include:
- The development of new protecting groups that are more selective and easier to de-protect.
- The development of new de-protection strategies that are more efficient and less harmful to peptides.
- The development of new methods for the temporary protection of amino groups in solid-phase peptide synthesis.
These advances have made it possible to synthesize peptides more efficiently and with higher yields. They have also made it possible to synthesize peptides that are more complex and that contain amino acids with sensitive side chains.
Conclusion
Amino group protection is an essential part of peptide synthesis. By using the appropriate protective groups and de-protection strategies, chemists can synthesize peptides with high yields and purity. Advances in amino group protection techniques have made it possible to synthesize more complex and sensitive peptides than ever before.