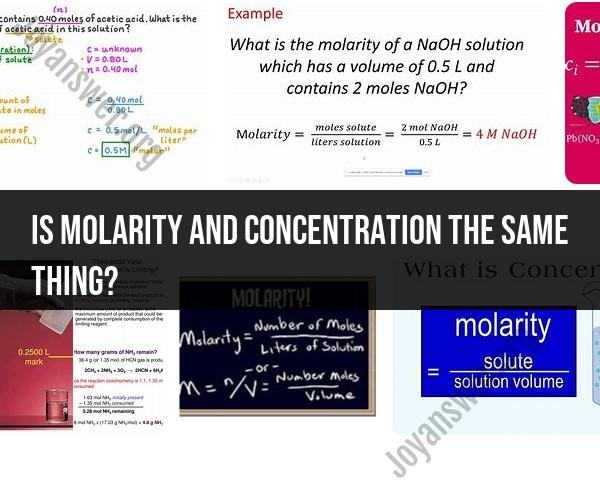
Is molarity and concentration the same thing?
Molarity and concentration are related concepts, but they are not the same thing. They refer to different ways of expressing the amount of a substance dissolved in a solution. Here's how they differ:
Molarity (M):
- Molarity is a measure of the concentration of a solute (substance being dissolved) in a solution.
- It is defined as the number of moles of solute per liter of solution.
- The formula for calculating molarity is: Molarity (M) = Moles of Solute (mol) / Volume of Solution (L).
- Molarity is expressed in units of moles per liter (mol/L or M).
Concentration:
- Concentration is a more general term that can refer to various ways of expressing the amount of a substance in a solution.
- It can be expressed in different units, such as mass/volume (e.g., grams per liter), percent concentration (%), parts per million (ppm), or parts per billion (ppb).
- Concentration does not necessarily specify the number of moles of solute; it can refer to the quantity of solute in any suitable unit, including mass or volume.
- Different types of concentration include mass concentration, volume concentration, and mole fraction, among others.
In summary, molarity specifically refers to the concentration of a solute in a solution and is expressed in moles per liter. Concentration, on the other hand, is a more general term that can encompass various units and ways of expressing the amount of a substance in a solution, not limited to moles per liter. When discussing solutions in a chemistry context, molarity is a common and precise way to express concentration. However, in other contexts, concentration can take on different forms, such as mass concentration (e.g., grams per liter) in a biology or environmental science context.
Molarity vs. Concentration: Understanding the Distinction in Chemistry
Molarity and concentration are two closely related concepts in chemistry, but they are not the same.
Concentration is a general term used to describe the amount of a substance dissolved in a solution. It can be expressed in a variety of ways, such as mass per volume (g/L), mole fraction, or molarity.
Molarity is a specific type of concentration that is expressed in moles per liter of solution (mol/L). It is the most common way to express concentration in chemistry.
To calculate the molarity of a solution, divide the number of moles of solute by the volume of solution in liters.
Molarity = moles of solute / liters of solution
For example, a 1 molar solution of sodium chloride (NaCl) contains 1 mole of NaCl dissolved in 1 liter of solution.
Concentration and Molarity: Different Measures of Solution Strength
Concentration and molarity are both measures of solution strength, but they differ in how they are expressed.
Concentration can be expressed in a variety of ways, such as mass per volume (g/L), mole fraction, or molarity. Molarity is always expressed in moles per liter of solution (mol/L).
Concentration is a more general term, while molarity is a more specific type of concentration.
Demystifying Molarity and Concentration: Key Concepts in Chemistry
Molarity and concentration are key concepts in chemistry. They are used to calculate the amount of solute in a solution, to prepare solutions of a specific concentration, and to understand the chemical behavior of solutions.
Here are some important things to remember about molarity and concentration:
- Concentration is a general term used to describe the amount of a substance dissolved in a solution.
- Molarity is a specific type of concentration that is expressed in moles per liter of solution (mol/L).
- To calculate the molarity of a solution, divide the number of moles of solute by the volume of solution in liters.
- Concentration and molarity are both measures of solution strength, but they differ in how they are expressed.