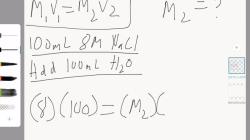How do you calculate a 1 10 dilution?
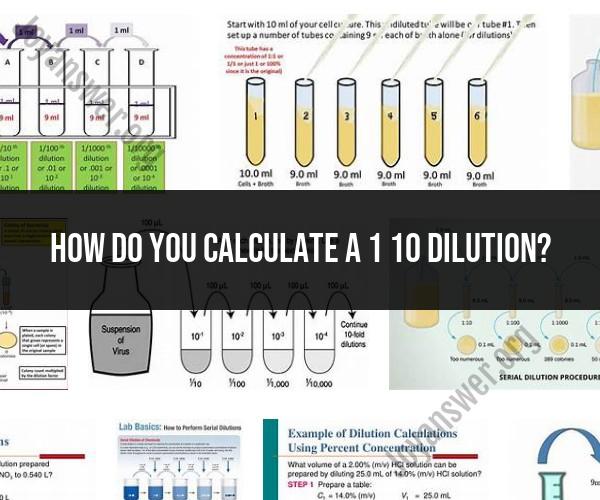
Understanding and mastering the concept of dilutions is crucial in various scientific and laboratory settings. A common dilution ratio is 1:10, where a concentrated solution is diluted to one-tenth of its original concentration. Here's a comprehensive guide to help you master the art of 1:10 dilution calculations:
1. Understanding the Dilution Ratio:
In a 1:10 dilution, one part of the concentrated solution is mixed with nine parts of the diluent (usually a solvent, such as water). This results in a total of ten parts, where the original concentration is divided by ten.
2. Calculating the Dilution Factor:
The dilution factor represents the ratio of the volume of the diluted solution to the volume of the original solution. For a 1:10 dilution, the dilution factor is 10, as the solution is diluted tenfold.
3. Calculating the Final Concentration:
To calculate the final concentration of the diluted solution, divide the original concentration by the dilution factor. This gives you the new concentration after dilution.
4. Example Calculation:
Suppose you have a solution with a concentration of 200 mg/mL and you want to prepare a 1:10 dilution:
Original Concentration: 200 mg/mL
Dilution Factor: 10
Final Concentration: 200 mg/mL ÷ 10 = 20 mg/mL
5. Real-World Applications:
1:10 dilutions are commonly used in scientific research, medical laboratories, and various industries to adjust concentrations for experiments, tests, and analyses.
Conclusion:
Mastering the art of 1:10 dilution calculations is an essential skill for accurately preparing solutions with specific concentrations. By understanding the dilution ratio, calculating the dilution factor, and determining the final concentration, you'll be well-equipped to perform dilutions effectively in a wide range of scientific and practical contexts.