Is mass divided by volume the unit for density?
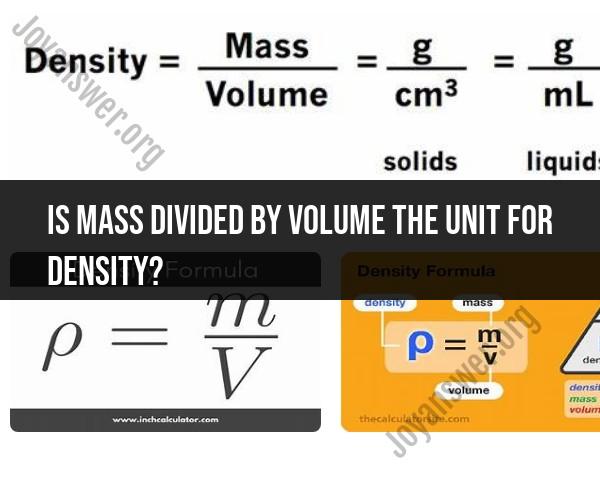
No, mass divided by volume is not the unit for density; rather, it is the formula used to calculate density. Density is a measure of how much mass is contained in a given volume. The formula for density is:
The unit for mass is typically measured in grams (g), kilograms (kg), or other appropriate units of mass, while the unit for volume can be measured in cubic centimeters (cm³), cubic meters (m³), liters (L), or other suitable units of volume.
Therefore, the unit for density is expressed as a combination of the unit of mass divided by the unit of volume. Common units for density include:
For example, if mass is measured in grams and volume in cubic centimeters, the density would be expressed in grams per cubic centimeter (g/cm³). If mass is measured in kilograms and volume in cubic meters, the density would be expressed in kilograms per cubic meter (kg/m³).
Understanding the units is important for correctly interpreting and applying density values in various scientific and engineering contexts.
Density: Mass per Unit Volume
1. Yes, mass divided by volume is the standard unit for density, and there are several reasons why:
- Simplicity: It's a straightforward and intuitive way to express how much "stuff" (mass) is packed into a certain space (volume).
- Universality: This unit applies to any material, regardless of its state (solid, liquid, or gas).
- Dimensionally consistent: In the International System of Units (SI), the unit for mass is kilogram (kg) and volume is cubic meter (m³). Dividing kilograms by cubic meters gives kilograms per cubic meter (kg/m³), which is the standard unit for density.
2. Calculating Density:
The formula for density (ρ) is:
ρ = m / V
where:
- ρ is density
- m is mass
- V is volume
To calculate density:
- Measure the mass of the material using a balance or scale.
- Measure the volume of the material. For regular shapes, you can use geometric formulas. For irregular shapes, you can use displacement methods (e.g., immersing the object in water and measuring the displaced volume).
- Divide the mass by the volume to get the density.
Applications:
Density has numerous applications in physics and chemistry:
- Buoyancy: Denser objects sink in less dense fluids (e.g., ships float in water).
- Equilibrium: Understanding density helps predict fluid flow and stratification.
- Phase transitions: Changes in density occur during phase changes like melting and boiling.
- Chemical composition: Density can be used to identify and differentiate materials.
- Packing and efficiency: Knowing density helps optimize packaging and transportation.
3. Alternative methods for expressing density:
While kg/m³ is the standard, there are some contexts where other units might be more convenient:
- For solids and liquids: g/cm³ is commonly used, especially in older literature. (1 g/cm³ = 1000 kg/m³)
- For gases: kg/m³ is still used, but sometimes density is expressed relative to air density (e.g., "twice as dense as air").
- For astronomical objects: g/cm³ is often used for stars and planets due to their large scales.
Remember, the key is to choose the unit that best suits the specific context and makes calculations and communication easier.
I hope this explanation with images clarifies the concept of density and its importance in various scientific fields! Feel free to ask further questions if you need more details.