How do I calculate molarity/concentration?
Calculating molarity, also known as concentration, involves determining the number of moles of a solute (substance being dissolved) in a given volume of solution. The formula for calculating molarity is:
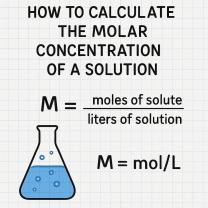
Molarity (M) = Moles of Solute (n) / Volume of Solution (V) in liters
Here's a step-by-step guide on how to calculate molarity:
Gather Information:
- Determine the following:
- The amount of solute in grams (g).
- The molecular weight (molar mass) of the solute in grams per mole (g/mol).
- The volume of the solution in liters (L).
- Determine the following:
Convert Mass to Moles:
- Calculate the number of moles of the solute by dividing the mass (in grams) by the molar mass (in g/mol).
- n (moles) = Mass (g) / Molar Mass (g/mol)
- Calculate the number of moles of the solute by dividing the mass (in grams) by the molar mass (in g/mol).
Convert Milliliters to Liters (if needed):
- Ensure that the volume of the solution is in liters. If the volume is given in milliliters (mL), divide by 1000 to convert it to liters:
- V (liters) = Volume (mL) / 1000
- Ensure that the volume of the solution is in liters. If the volume is given in milliliters (mL), divide by 1000 to convert it to liters:
Calculate Molarity:
- Finally, use the formula to calculate molarity by dividing the number of moles of solute by the volume of the solution in liters.
- Molarity (M) = Moles of Solute (n) / Volume of Solution (V)
- Finally, use the formula to calculate molarity by dividing the number of moles of solute by the volume of the solution in liters.
Let's illustrate this process with an example:
Example: Calculate the molarity of a solution containing 2 grams of sodium chloride (NaCl) dissolved in 500 mL of water.
Gather Information:
- Mass of NaCl (solute) = 2 grams
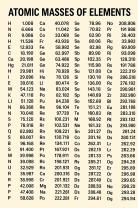
- Molar mass of NaCl = 58.44 g/mol (sodium: 22.99 g/mol, chlorine: 35.45 g/mol)
- Volume of the solution = 500 mL = 0.5 L
Convert Mass to Moles:
- Calculate the moles of NaCl:
- n (moles) = Mass (g) / Molar Mass (g/mol)
- n = 2 g / 58.44 g/mol ≈ 0.0342 moles
- Calculate the moles of NaCl:
Convert Milliliters to Liters:
- The volume of the solution is given in milliliters, so convert it to liters:
- V (liters) = Volume (mL) / 1000
- V = 500 mL / 1000 = 0.5 L
- The volume of the solution is given in milliliters, so convert it to liters:
Calculate Molarity:
- Use the formula to calculate molarity:
- Molarity (M) = Moles of Solute (n) / Volume of Solution (V)
- Molarity (M) = 0.0342 moles / 0.5 L = 0.0684 M
- Use the formula to calculate molarity:
So, the molarity of the NaCl solution is approximately 0.0684 M. This means that there are 0.0684 moles of NaCl dissolved in every liter of the solution.
How to Calculate Molarity: Step-by-Step Guide to Concentration Determination
Molarity is a measure of the concentration of a solution, defined as the number of moles of solute per liter of solution. It is a useful unit of concentration for many chemical and biochemical applications.
To calculate molarity, you will need to know the following information:
- The mass of the solute in grams
- The molar mass of the solute
- The volume of the solution in liters
Once you have this information, you can use the following formula to calculate molarity:
Molarity (M) = moles of solute / liters of solution
Step-by-Step Guide to Calculating Molarity:
- Weigh the solute in grams.
- Calculate the moles of solute by dividing the mass of the solute by its molar mass.
- Measure the volume of the solution in liters.
- Divide the moles of solute by the liters of solution to calculate molarity.
Example:
Calculate the molarity of a solution that contains 10.0 grams of sodium chloride (NaCl) dissolved in 500.0 milliliters (mL) of water.
- The molar mass of NaCl is 58.44 grams per mole.
- Moles of NaCl = 10.0 grams / 58.44 grams per mole = 0.171 moles
- 500.0 mL is equal to 0.500 liters.
- Molarity = 0.171 moles / 0.500 liters = 0.342 M
Molarity and Concentration Calculations: The Essentials for Chemistry
Molarity is a fundamental concept in chemistry, and it is used in many different calculations. For example, molarity can be used to calculate the following:
- The amount of solute in a solution
- The volume of solution required to dissolve a certain amount of solute
- The concentration of a solution after dilution or mixing
Math Behind Molarity: How to Compute Concentration with Precision
The math behind molarity is relatively straightforward. However, there are a few things to keep in mind when calculating molarity with precision.
- Make sure to use accurate measurements for the mass of the solute and the volume of the solution.
- Use the correct molar mass for the solute.
- Be careful when converting between units. For example, 1 liter is equal to 1000 milliliters.
By following these tips, you can calculate molarity with precision and accuracy.