How to calculate dilution rate?
The dilution rate measures the extent to which a solution has been diluted by another substance, typically a solvent or another solution. The formula to calculate dilution rate depends on the initial and final volumes and concentrations of the substances involved. Here's a step-by-step guide:
1. Understand the Components:
- Initial Solution: The concentration and volume of the initial (stock) solution.
- Diluted Solution: The concentration and volume of the final diluted solution after dilution.
2. Use the Dilution Formula:
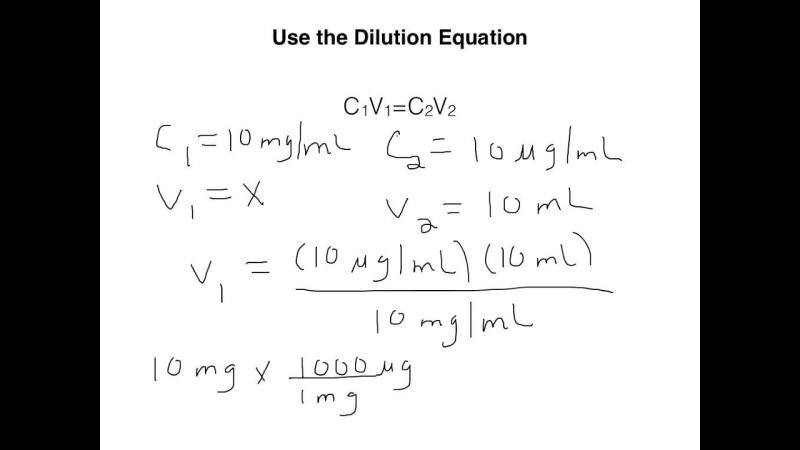
- The dilution formula is:
- Where:
- = Initial concentration of the solution.
- = Initial volume of the solution.
- = Final concentration of the solution after dilution.
- = Final volume of the solution after dilution.
3. Calculate Dilution Rate:
- Rearrange the dilution formula to solve for the dilution rate or final concentration ().
- The formula for dilution rate after rearranging the equation is:
4. Plug in the Values:
- Input the known values into the formula.
- If you know the initial concentration () and volume () of the stock solution and the final volume () of the diluted solution, use the formula to calculate the final concentration ().
5. Solve for the Dilution Rate:
- Perform the calculations using the given values to find the final concentration (), which represents the dilution rate after the solution has been diluted.
Example:Suppose you have a 100 mL stock solution with a concentration of 0.2 M. You dilute this solution by adding water to reach a final volume of 500 mL. To find the final concentration after dilution ():
In this example, after dilution, the final concentration of the solution () is 0.04 M.
This calculation method helps determine the dilution rate or final concentration of a solution after diluting it with another substance or solvent. Adjust the formula based on the known values to solve for the unknown variable.
1. How to Compute the Dilution Rate
The dilution rate is a ratio that represents the proportion of solute (the substance being diluted) to the solvent (the diluting substance). It is typically expressed as a ratio of solute to solvent volumes. For example, a dilution rate of 1:10 means that one part of solute is mixed with ten parts of solvent.
2. Steps Involved in Calculating the Dilution Rate
To calculate the dilution rate, follow these steps:
Determine the initial volume of the solute (V₀).
Determine the final volume of the diluted solution (V₁).
Calculate the dilution factor (DF) using the formula:
DF = V₁ / V₀
- Express the dilution rate as a ratio of solute to solvent volumes.
For example, if you have 100 mL of a solute and you mix it with 900 mL of solvent to create a 1000 mL solution, the dilution rate would be:
DF = 1000 mL / 100 mL = 10
Dilution rate = 1:10 (1 part solute to 10 parts solvent)
3. Specific Formulas or Methods for Determining Dilution Rates
There are two main formulas for determining dilution rates:
- Formula for dilution of a solution:
M₁V₁ = M₂V₂
where:
- M₁ is the initial concentration of the solute
- V₁ is the initial volume of the solute
- M₂ is the final concentration of the solute
- V₂ is the final volume of the diluted solution
- Formula for dilution factor:
DF = V₂ / V₀
where:
- DF is the dilution factor
- V₁ is the final volume of the diluted solution
- V₀ is the initial volume of the solute