What is an ICE table and how is it used to calculate equilibrium concentration?
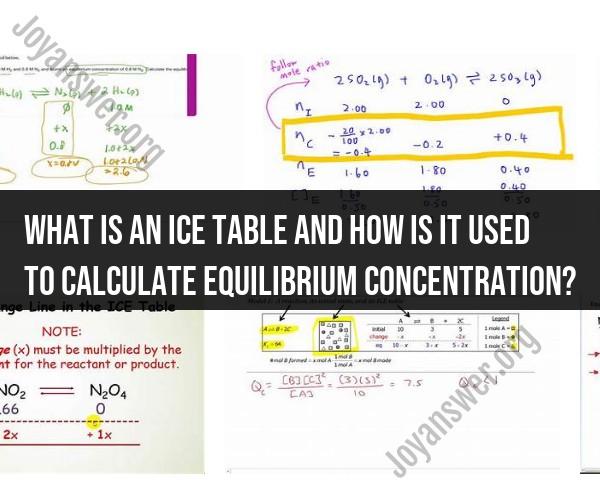
An ICE table is a useful tool in chemistry used to calculate the equilibrium concentrations of reactants and products in a chemical reaction. "ICE" stands for "Initial," "Change," and "Equilibrium," which are the key components of the table. It's commonly used in equilibrium calculations, especially for problems involving the concentrations of substances at equilibrium.
Here's how an ICE table works and how it's used to calculate equilibrium concentrations:
1. Initial (I): In this column, you start by writing down the initial concentrations of all the reactants and products before any reaction occurs. These initial concentrations are typically provided in the problem or are known from experimental conditions.
2. Change (C): In this column, you determine how the concentrations change as the reaction proceeds. To do this, consider the stoichiometry of the reaction (coefficients of the balanced chemical equation). You'll use the coefficients to determine how much of each substance is consumed or produced as the reaction progresses.
- For reactants, you subtract the amount consumed (according to the stoichiometry) from the initial concentration.
- For products, you add the amount produced (according to the stoichiometry) to the initial concentration.
3. Equilibrium (E): In this column, you calculate the equilibrium concentrations by adding the changes to the initial concentrations. These equilibrium concentrations represent the final concentrations of all substances when the reaction reaches equilibrium.
Here's a step-by-step example:
Let's say you have the reaction: A + 2B ⇌ 3C.
Initial concentrations (I):
- [A] = 0.1 M
- [B] = 0.2 M
- [C] = 0 M (assuming no C initially)
Change (C):
- For A: -x (because it reacts)
- For B: -2x (because it reacts)
- For C: +3x (because it's produced)
Equilibrium (E):
- [A] = 0.1 - x
- [B] = 0.2 - 2x
- [C] = 3x (assuming it's produced)
At equilibrium, you'll typically have an equilibrium expression (equilibrium constant, K) that relates the concentrations of the substances involved. You can use this expression, along with the ICE table, to calculate the value of x and find the equilibrium concentrations of all species.
Once you find the value of x, you can substitute it back into the equilibrium expressions for each substance to determine their equilibrium concentrations.
The ICE table is a systematic way to solve equilibrium problems and find the concentrations of substances at equilibrium by considering the stoichiometry of the reaction and the initial conditions.