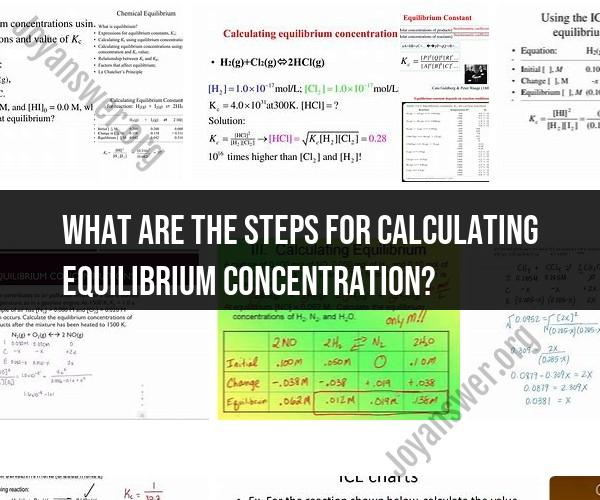
What are the steps for calculating equilibrium concentration?
Calculating equilibrium concentration in a chemical reaction involves using the principles of equilibrium constants and the initial concentrations of reactants and products. Here are the step-by-step instructions for calculating equilibrium concentration:
Write the Balanced Chemical Equation:Start by writing the balanced chemical equation for the reaction of interest. Ensure that it's balanced in terms of the number of atoms for each element on both the reactant and product sides.
Write the Equilibrium Expression:Write the equilibrium expression (also known as the equilibrium constant expression) for the reaction. The general form is:
Where:
- is the equilibrium constant.
- , , , and are the concentrations of the reactants and products.
- , , , and are the coefficients of the balanced chemical equation for reactants and products, respectively.
Determine Initial Concentrations:Gather information about the initial concentrations of the reactants and products. These are typically given in the problem or experiment.
Set Up the Equilibrium Expression:Plug in the initial concentrations of the reactants and products into the equilibrium expression:
Where:
- , , , and are the initial concentrations of the reactants and products.
Solve for Unknown Concentrations:You might be asked to find the equilibrium concentrations of certain species (, , etc.). To do this, rearrange the equilibrium expression and solve for the unknown concentrations:
Substituting the values of , , , and from your problem into this equation will give you the equilibrium concentration of .
Units and Significant Figures:Pay attention to units and significant figures. Ensure that all concentrations have the same units, and round your answer to an appropriate number of significant figures based on the given data.
Check for Validity:After calculating the equilibrium concentrations, check if they make sense physically and chemically. Concentrations cannot be negative, and they should satisfy the equilibrium expression when substituted back into it.
Finalize the Answer:Present your final equilibrium concentrations for the reactants and products, making sure they are in the correct units and format.
These steps are a general guideline for calculating equilibrium concentrations in a chemical reaction. The specific approach may vary depending on the problem and the information provided. Always follow the principles of equilibrium and use the appropriate equilibrium constant expression for the reaction you're dealing with.