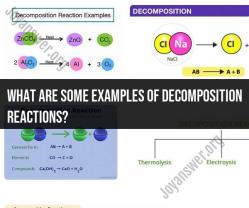
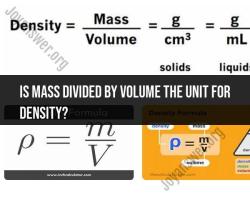
How do you find the surface area of a particle?
The surface area of particles plays a crucial role in various scientific disciplines, from chemistry to material science. Understanding how to measure and interpret surface area is essential for comprehending the behavior and properties of particles at the microscale. In this article, we'll explore the measurement methods and significance of particle surface area.
Measurement Methods
There are several techniques for measuring the surface area of particles, depending on their size and characteristics:
1. BET (Brunauer-Emmett-Teller) Method
The BET method is commonly used for porous materials and involves adsorbing a gas onto the particle's surface. By analyzing the gas adsorption isotherm, the specific surface area can be determined.
2. Gas Adsorption Techniques
Gas adsorption methods, such as nitrogen adsorption, help estimate the surface area by measuring the amount of gas adsorbed onto the particles.
3. Micromeritics Analysis
Micromeritics analysis involves using various instruments to measure particle size distribution, including the surface area of the particles.
Significance of Particle Surface Area
The surface area of particles holds significant implications across scientific and industrial domains:
1. Chemical Reactivity
Particles with larger surface areas are more chemically reactive due to increased exposure of active sites. This is vital in catalytic processes and chemical reactions.
2. Adsorption and Absorption
Particle surface area affects adsorption and absorption phenomena, such as gas adsorption onto activated carbon or drug absorption in pharmaceuticals.
3. Material Properties
The surface area influences material properties like porosity, permeability, and mechanical strength. Materials with higher surface areas may exhibit improved characteristics.
4. Environmental Applications
Particle surface area is important in environmental contexts, such as pollutant adsorption onto surfaces or soil-water interactions.
5. Nanotechnology
In nanotechnology, nanoparticles' unique properties are often attributed to their high surface area, impacting applications like drug delivery and sensors.
Conclusion
Measuring and understanding the surface area of particles is essential for gaining insights into their behavior and properties. From chemical reactions to environmental processes, particle surface area plays a pivotal role in numerous scientific endeavors, contributing to advancements in various fields.