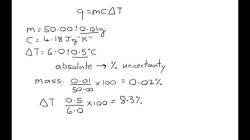What is the density of water at specific temperature?
The density of water changes with temperature due to its unique molecular structure. As water molecules heat up, they gain energy and move farther apart, which causes a decrease in density. Conversely, as water cools, its molecules slow down and come closer together, leading to an increase in density.
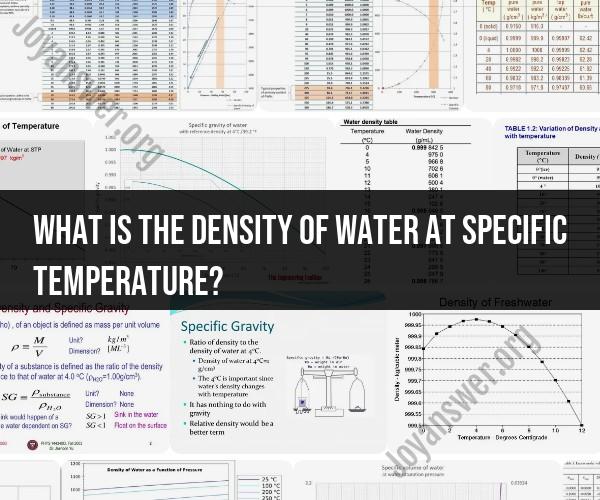
Here are some reference values for the density of water at specific temperatures:
Note: These values are approximate and based on standard conditions (normal atmospheric pressure of 1 atmosphere or 101.325 kPa).
0°C (32°F): The density of water at its maximum density point (4°C) is approximately 999.84 kg/m³. However, at 0°C, the density is slightly higher, around 999.97 kg/m³.
4°C (39.2°F): Water reaches its maximum density at around 4°C. At this temperature, the density is approximately 1000 kg/m³.
20°C (68°F): The density of water at 20°C is about 998.21 kg/m³.
25°C (77°F): At 25°C, the density of water is approximately 997.07 kg/m³.
100°C (212°F): The density of water at its boiling point of 100°C is about 958.4 kg/m³.
Remember that these values are approximate and can vary slightly due to factors such as pressure, impurities, and isotopic composition. The density of water is commonly used as a reference for various scientific and engineering calculations, particularly in fields like fluid mechanics and thermodynamics.