What is the speed of the de Broglie wave?
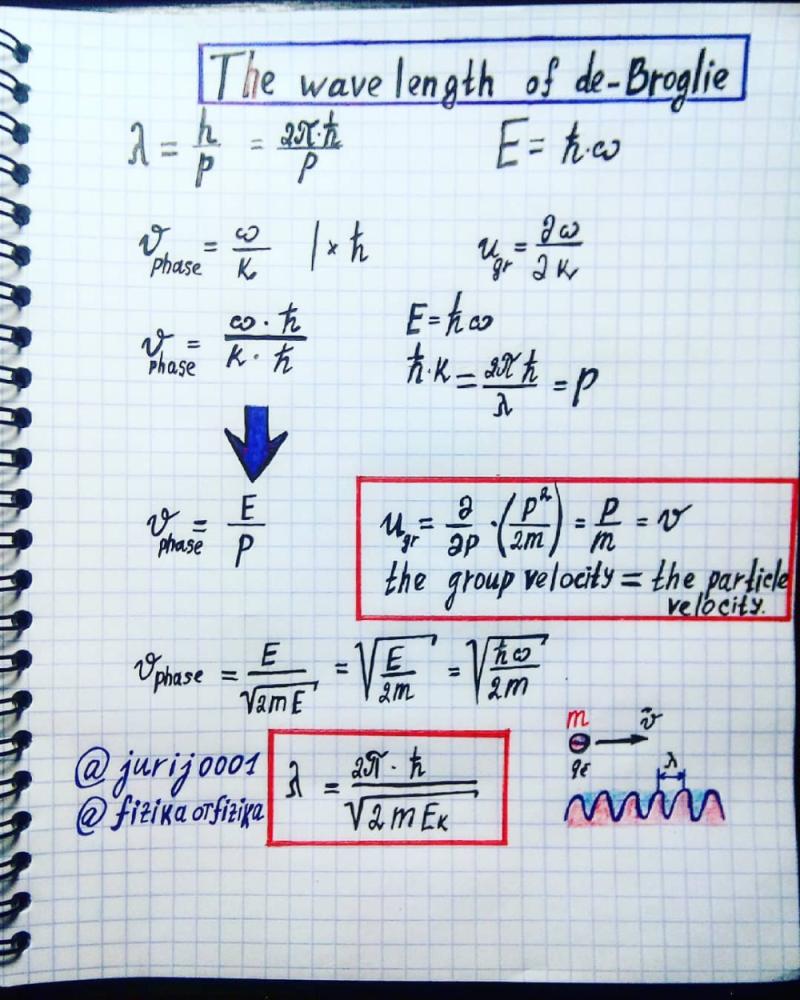
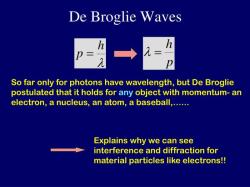
The de Broglie wavelength is a concept in quantum mechanics that is associated with the wave-particle duality of matter. According to Louis de Broglie's hypothesis, particles such as electrons exhibit both wave-like and particle-like properties. The de Broglie wavelength (λ) is given by the equation:
where:
- is the de Broglie wavelength,
- is Planck's constant (),
- is the momentum of the particle.
The speed () of a de Broglie wave is related to the wavelength and the particle's mass () through the equation:
This equation indicates that the speed of a de Broglie wave is inversely proportional to the product of the particle's mass and its de Broglie wavelength. As the mass of the particle increases, the speed of the associated de Broglie wave decreases.
It's important to note that the de Broglie wavelength and the associated wave nature of particles are significant at the quantum scale. For macroscopic objects, the de Broglie wavelength is exceedingly small, and the classical laws of physics are typically sufficient to describe their behavior. The wave-particle duality becomes more apparent and relevant when dealing with particles at the atomic and subatomic levels.
The formula for calculating the speed of a de Broglie wave is:
λ = h/p
where:
- λ is the wavelength of the de Broglie wave
- h is Planck's constant (6.63 × 10^-34 J·s)
- p is the momentum of the particle
The momentum of a particle is given by:
p = mv
where:
- m is the mass of the particle
- v is the velocity of the particle
Therefore, the formula for calculating the speed of a de Broglie wave can also be written as:
λ = h/mv
Yes, the mass and velocity of a particle do relate to its de Broglie wavelength. The de Broglie wavelength is inversely proportional to the momentum of the particle. This means that the wavelength of a particle decreases as its momentum increases. The de Broglie wavelength is also inversely proportional to the mass of the particle. This means that the wavelength of a particle decreases as its mass increases.
For example, the de Broglie wavelength of an electron is much smaller than the de Broglie wavelength of a proton. This is because the electron is much lighter than the proton. The de Broglie wavelength of a particle can be very small, even for macroscopic objects. For example, the de Broglie wavelength of a baseball is about 10^-34 meters. This is much smaller than the size of the baseball, which is about 0.07 meters.
The de Broglie wavelength is a fundamental concept in quantum mechanics. It has many important implications, including the wave-particle duality of matter.