What does de Broglie wavelength mean?
The de Broglie wavelength is a concept in quantum mechanics that describes the wavelength associated with a particle, particularly matter waves. It is named after Louis de Broglie, a French physicist who proposed the idea in 1924 as part of his doctoral thesis.
The key insight of de Broglie was that if light, traditionally thought of as waves, could also exhibit particle-like behavior (as demonstrated by the photoelectric effect), then perhaps particles like electrons could exhibit wave-like behavior. He suggested that any particle with momentum (including electrons, protons, and even macroscopic particles under certain conditions) should be associated with a wavelength.
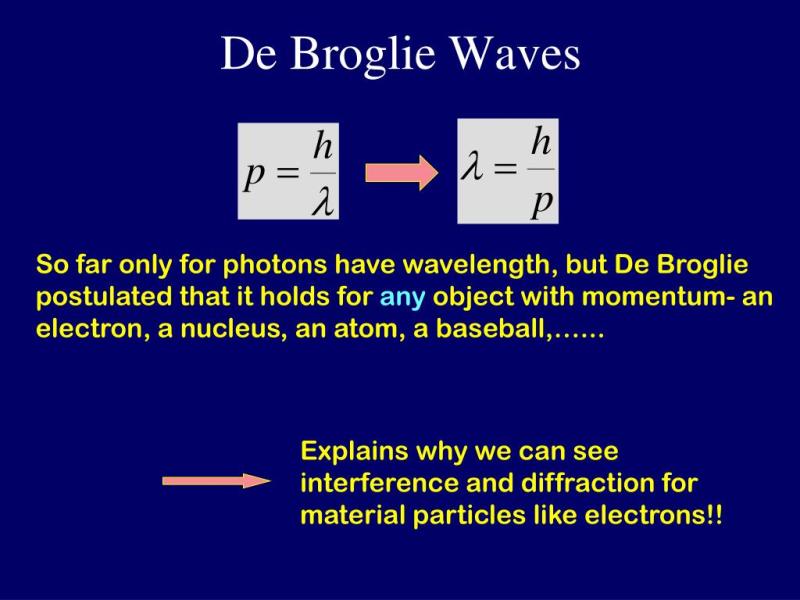
The de Broglie wavelength () is given by the following formula:
where:
- is the de Broglie wavelength,
- is Planck's constant (),
- is the momentum of the particle.
This equation suggests that as the momentum of a particle decreases, its de Broglie wavelength increases. In practical terms, this means that particles with higher momentum (e.g., high-speed electrons) have shorter de Broglie wavelengths, and particles with lower momentum (e.g., slower electrons) have longer de Broglie wavelengths.
The wave-particle duality concept, which is central to quantum mechanics, implies that particles can exhibit both wave-like and particle-like properties, depending on the experimental conditions. The de Broglie wavelength is a fundamental aspect of this duality, providing a way to relate the classical concept of momentum to the quantum mechanical concept of wavelength.
What is the significance of the de Broglie wavelength?
The de Broglie wavelength is a fundamental concept in quantum mechanics that has far-reaching implications for our understanding of the physical world. It is a consequence of wave-particle duality, the principle that all particles exhibit both wave-like and particle-like properties. The de Broglie wavelength is the wavelength associated with a particle, and it is related to the particle's momentum by the equation:
λ = h/p
where:
- λ is the de Broglie wavelength
- h is the Planck constant
- p is the particle's momentum
The de Broglie wavelength has a number of important implications, including:
- It provides a link between the wave and particle aspects of matter. This connection is one of the most profound insights of quantum mechanics, and it has led to a deeper understanding of the nature of matter.
- It explains the quantization of energy levels in atoms. The quantization of energy levels is one of the most important features of atomic structure, and it is directly related to the de Broglie wavelength of electrons.
- It has applications in a number of fields, including electronics, optics, and nanotechnology. For example, the de Broglie wavelength is used to design electron microscopes, which are capable of imaging objects with atomic resolution.
The de Broglie wavelength is a key concept in quantum mechanics, and it has helped us to develop a more complete understanding of the physical world.