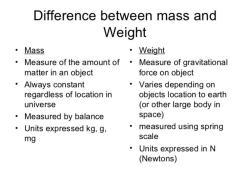
What is the relation between weight and volume?
The relationship between weight and volume is influenced by the density of a substance. Density is a fundamental property of matter that relates the mass (weight) of an object to its volume. In simple terms, density is a measure of how much mass is contained in a given volume. The formula for density is:
Density (D) = Mass (M) / Volume (V)
Here's how the relationship between weight (mass) and volume works:
High Density: When a substance has a high density, it means that a significant amount of mass is concentrated in a relatively small volume. This results in a high weight relative to the volume. For example, metals like lead and gold have high densities.
Low Density: When a substance has a low density, it means that there is relatively little mass in a relatively large volume. This results in a low weight relative to the volume. For example, materials like cotton or foam have low densities.
Constant Density: If the density of a substance is constant, then the relationship between weight and volume is linear. If you double the volume of a substance with constant density, you will also double the weight. This is because density remains the same.
In practical terms, when dealing with everyday materials and objects, the relationship between weight and volume can vary significantly. For example, a piece of wood may have a different weight and volume compared to a piece of plastic of the same size. This variation occurs because different materials have different densities.
It's important to note that the units of measurement used for weight and volume can affect the numerical relationship between them. For example, in the International System of Units (SI), weight is typically measured in kilograms (kg) and volume in cubic meters (m^3), which results in density being expressed in kg/m^3. In common usage, weight is often measured in grams (g) and volume in milliliters (mL) or liters (L).
In summary, the relationship between weight and volume is determined by the density of a substance. Higher density corresponds to greater weight for a given volume, while lower density corresponds to less weight for the same volume. The specific numerical relationship depends on the density of the material and the units of measurement used.
Understanding the Relationship Between Weight and Volume
Weight and volume are two different physical properties of matter. Weight is the force of gravity acting on an object, while volume is the amount of space that an object occupies.
The relationship between weight and volume is described by the following equation:
Weight = Density × Volume
Where:
- Weight is measured in units of force, such as newtons (N) or pounds-force (lbf)
- Density is measured in units of mass per unit volume, such as kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³)
- Volume is measured in units of volume, such as cubic meters (m³) or cubic feet (ft³)
This equation tells us that the weight of an object is directly proportional to its density and volume. In other words, the denser an object is, the heavier it will be for a given volume. Conversely, the larger the volume of an object is, the heavier it will be for a given density.
The Role of Density in Weight-Volume Relationships
Density is a key factor in determining the weight-volume relationship of an object. Density is a measure of how much mass is packed into a given volume. The higher the density of an object, the more mass it has per unit volume. This means that denser objects will weigh more than less dense objects of the same volume.
For example, a block of lead has a higher density than a block of wood of the same size. This means that the block of lead will weigh more than the block of wood, even though they have the same volume.
Measuring Weight and Volume in Different Units
Weight and volume can be measured in a variety of different units. The most common units for weight are newtons (N) and pounds-force (lbf). The most common units for volume are cubic meters (m³) and cubic feet (ft³).
To convert between different units of weight and volume, you can use the following conversion factors:
- 1 N = 0.2248 lbf
- 1 lbf = 4.448222 N
- 1 m³ = 35.3147 ft³
- 1 ft³ = 0.0283168 m³
Practical Examples of Weight-Volume Calculations
Weight-volume calculations are used in a variety of practical applications. For example, weight-volume calculations are used to:
- Determine the shipping weight of a package
- Calculate the weight of a load of cargo
- Design storage tanks and silos
- Determine the amount of fuel in a tank
- Calculate the buoyancy of a ship
Factors Affecting Weight and Volume in Various Materials
The weight and volume of various materials can be affected by a number of factors, including:
- Temperature: The temperature of a material can affect its density. For example, metals expand when they are heated and contract when they are cooled. This means that the density of a metal will change with temperature.
- Pressure: The pressure on a material can also affect its density. For example, water is more dense at high pressures than at low pressures. This is because the high pressure forces the water molecules closer together.
- Impurities: The presence of impurities can also affect the density of a material. For example, gold with impurities will be less dense than pure gold.
- Porosity: The porosity of a material is the amount of empty space in the material. Porous materials have a lower density than non-porous materials. For example, a sponge has a lower density than a solid block of material.
By understanding the relationship between weight and volume, we can make better decisions about how to design, build, and use materials.