How does water evaporate completely when at room temperature?
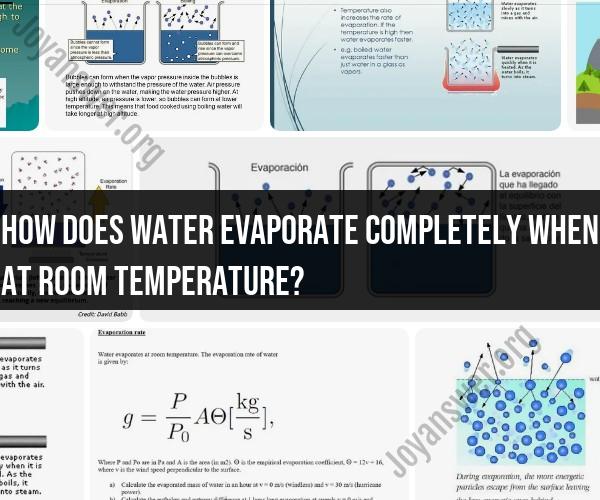
Water evaporates at room temperature due to the kinetic energy of its molecules. Even at temperatures below boiling point, some water molecules possess enough energy to transition from the liquid phase to the gas phase. Here's an explanation of the process:
Molecular Motion: In any substance, including water, molecules are in constant motion due to thermal energy. This motion is known as kinetic energy. In the liquid state, water molecules are attracted to each other by cohesive forces, but they still have enough energy to move around.
Escape from Surface: At the surface of the water, some molecules gain enough energy to break free from the cohesive forces that hold them in the liquid state. These molecules become water vapor in the air.
Evaporation: Evaporation is the process by which the more energetic water molecules leave the liquid phase and enter the gaseous phase. This occurs throughout the liquid, not just at the surface. However, the rate of evaporation is faster at the surface.
Dynamic Equilibrium: As water molecules continue to evaporate, there is also a process called condensation happening concurrently. Water vapor molecules can lose energy and return to the liquid state. When the rate of evaporation equals the rate of condensation, a dynamic equilibrium is established, and the water appears to stop evaporating.
Factors influencing the rate of evaporation include:
Temperature: Higher temperatures provide more thermal energy to the water molecules, increasing the rate of evaporation.
Humidity: The air's humidity level affects the evaporation rate. In a dry environment, the air can accommodate more water vapor, facilitating faster evaporation. In a humid environment, where the air is already saturated with moisture, evaporation is slower.
Surface Area: Evaporation is faster from a larger surface area.
Air Movement: Wind or air currents can carry away water vapor from the surface, promoting faster evaporation.
In summary, even at room temperature, some water molecules have enough energy to transition into the gas phase, leading to the process of evaporation. This is a dynamic and continuous process that occurs as long as there is a source of liquid water and suitable environmental conditions.
Sure, here is the answer to your question:
Factors that contribute to the complete evaporation of water at room temperature:
- Temperature: The higher the temperature, the faster the rate of evaporation. This is because at higher temperatures, water molecules have more kinetic energy and are more likely to escape from the liquid phase and become gas molecules.
- Surface area: The larger the surface area of the water, the faster the rate of evaporation. This is because there are more water molecules at the surface that can escape into the air.
- Humidity: The lower the humidity, the faster the rate of evaporation. This is because there is less water vapor in the air to slow down the evaporation process.
- Wind speed: The higher the wind speed, the faster the rate of evaporation. This is because the wind helps to remove water vapor from the surface of the water, which creates a concentration gradient that drives further evaporation.
Does surface area affect the rate of evaporation of water at room temperature?
Yes, surface area does affect the rate of evaporation of water at room temperature. The larger the surface area, the faster the rate of evaporation. This is because there are more water molecules at the surface that can escape into the air. For example, a puddle of water will evaporate much faster than a pond of water, because the puddle has a larger surface area relative to its volume.