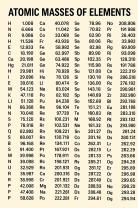
What is a 1 1000 dilution?
A 1:1000 dilution, in scientific terms, represents a specific ratio used to decrease the concentration of a substance in a solution. In this context, the first number (1) represents the amount of the substance you want to dilute, while the second number (1000) represents the total volume of the resulting solution. Here's a breakdown of what a 1:1000 dilution means:
Initial Substance: Let's say you have a concentrated solution of a substance (e.g., a chemical, a microorganism, or a compound) that you want to make less concentrated. The 1 in the ratio represents the amount of this initial substance that you'll use.
Total Volume: The 1000 in the ratio represents the total volume of the resulting diluted solution. This could be measured in any unit of volume, such as milliliters (mL), liters (L), or any other appropriate unit.
To perform a 1:1000 dilution, you would typically follow these steps:
Measure the amount of the initial substance that you want to dilute. For example, let's say you have 1 mL of a concentrated solution.
Prepare a container (e.g., a flask or beaker) with a total volume of 1000 mL (1 liter) of the diluent. The diluent is the liquid in which you're diluting the substance. This could be water, a buffer solution, or another appropriate solvent.
Carefully add the measured amount of the initial substance (e.g., 1 mL) to the container with the 1000 mL of diluent.
Mix the solution thoroughly to ensure uniform distribution of the substance within the diluent.
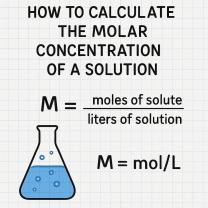
The result is a diluted solution in which the initial substance is now 1/1000th (or 0.1%) of its original concentration. In other words, you've reduced its concentration by a factor of 1000.
This type of dilution is commonly used in laboratory work, especially in microbiology and analytical chemistry, to create solutions with lower concentrations for various experiments and analyses. The specific dilution ratio you use depends on the requirements of your experiment or procedure.