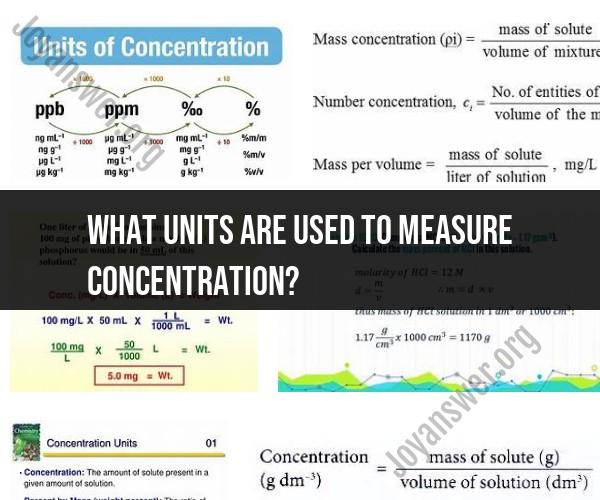
What units are used to measure concentration?
Concentration is a measure of the amount of a substance (solute) that is dissolved in a given volume of a solution. Different units of measurement for concentration are used depending on the nature of the solution and the specific application. Here are some common units of measurement for concentration:
Molarity (M):
- Molarity is one of the most widely used units of concentration in chemistry. It represents the number of moles of solute dissolved in one liter of solution. The unit is expressed as moles per liter (mol/L or M). For example, a 1 M solution of sodium chloride (NaCl) contains 1 mole of NaCl dissolved in 1 liter of water.
Molality (m):
- Molality is another concentration unit that expresses the amount of solute in relation to the mass of the solvent. It is defined as the number of moles of solute per kilogram (kg) of solvent. The unit is expressed as mol/kg. Molality is often used in colligative property calculations and is less temperature-dependent than molarity.
Mass Percent (% Mass):
- Mass percent is a unit of concentration that expresses the mass of the solute as a percentage of the total mass of the solution. It is calculated using the formula:
- For example, a 10% (w/v) solution of glucose contains 10 grams of glucose in 100 grams of the solution.
Volume Percent (% Volume):
- Volume percent is used to express the volume of the solute as a percentage of the total volume of the solution. It is often used in the context of solutions of liquids. The formula is:
Parts Per Million (ppm) and Parts Per Billion (ppb):
- These units are used to express very low concentrations of solutes in a solution. Parts per million represents one part of solute in one million parts of solution, and parts per billion represents one part of solute in one billion parts of solution. These units are commonly used in environmental and trace analysis.
Normality (N):
- Normality is a concentration unit that accounts for the number of reactive species (ions or equivalents) in a solution. It is expressed as the number of equivalents of solute per liter of solution. Normality is particularly useful in acid-base reactions and redox reactions.
Formal Concentration (C):
- Formal concentration is a unit used in the context of coordination compounds and complex ions. It represents the concentration of a specific ion within a complex or coordination compound. The formal concentration is based on the stoichiometry of the complex.
Mole Fraction (X):
- Mole fraction expresses the ratio of the number of moles of solute to the total number of moles in the solution. It is dimensionless and is often denoted as X.
The choice of concentration unit depends on the specific application and the type of substances involved. Chemists and scientists use the appropriate unit to accurately describe the concentration of solutes in various contexts, from laboratory experiments to industrial processes.
Units of Concentration Measurement: Exploring Common Metrics
There are many different units of concentration measurement, but some of the most common include:
- Molarity (M): Molarity is defined as the number of moles of solute per liter of solution. For example, a 1 M solution of NaCl contains 1 mole of NaCl per liter of solution.
- Molality (m): Molality is defined as the number of moles of solute per kilogram of solvent. For example, a 1 m solution of NaCl contains 1 mole of NaCl per kilogram of water.
- Mass percent (% m/m): Mass percent is defined as the mass of solute per 100 grams of solution. For example, a 10% m/m solution of NaCl contains 10 grams of NaCl per 100 grams of solution.
- Volume percent (% v/v): Volume percent is defined as the volume of solute per 100 milliliters of solution. For example, a 10% v/v solution of ethanol contains 10 milliliters of ethanol per 100 milliliters of solution.
- Parts per million (ppm): Parts per million is defined as the number of parts of solute per million parts of solution. For example, a 1 ppm solution of NaCl contains 1 part of NaCl per million parts of solution.
- Parts per billion (ppb): Parts per billion is defined as the number of parts of solute per billion parts of solution. For example, a 1 ppb solution of NaCl contains 1 part of NaCl per billion parts of solution.
Quantifying Substances: Understanding Concentration Measurement Units
Concentration measurement units are important for quantifying substances in solution. By knowing the concentration of a solution, we can determine the amount of solute in a given volume of solution. This information can be used for a variety of purposes, such as:
- To prepare solutions of a specific concentration: For example, if we need to prepare a 1 M solution of NaCl, we know that we need to dissolve 1 mole of NaCl in 1 liter of water.
- To perform chemical reactions: For example, if we want to perform a chemical reaction that requires 0.5 moles of NaCl, we know that we need to use 500 mL of a 1 M solution of NaCl.
- To analyze the composition of solutions: For example, if we want to determine the concentration of NaCl in a solution, we can use a variety of analytical techniques, such as titration or gravimetry.
Chemistry Metrics: The Language of Concentration
Concentration measurement units are the language of concentration chemistry. By understanding and using these units correctly, we can communicate effectively with other chemists and perform accurate calculations.
Here are some additional tips for understanding and using concentration measurement units:
- Be aware of the different types of concentration units. There are many different types of concentration units, so it is important to be aware of the different types and to use the correct type of unit for the specific situation.
- Be able to convert between different types of concentration units. Sometimes it is necessary to convert between different types of concentration units. For example, if a recipe calls for a solution of 10% m/m NaCl, but we only have a 1 M solution of NaCl, we need to be able to convert between the two units in order to prepare the solution correctly.
- Use the correct units in calculations. When performing calculations involving concentration, it is important to use the correct units. For example, if we are calculating the amount of solute in a given volume of solution, we need to use units of moles and liters.
By following these tips, you can develop a strong understanding of concentration measurement units and use them effectively in your chemistry studies and career.