What are two elements have the same atomic mass?
"Elements with Identical Atomic Masses: An Exploration"
In the realm of chemistry and the periodic table, elements are classified and organized based on their atomic number, which corresponds to the number of protons in the nucleus of an atom. While most elements have distinct atomic masses, there are a few instances where two different elements share the same atomic mass. This intriguing phenomenon is a result of the complexities of atomic structure and the way atomic masses are calculated.
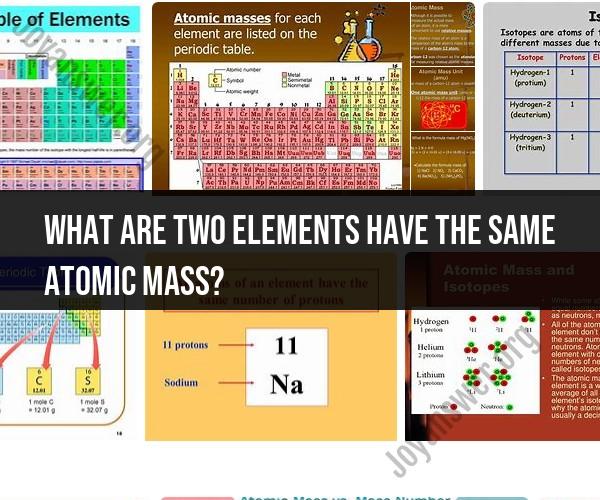
One prominent example of elements with identical atomic masses is carbon-12 (12C) and nitrogen-14 (14N). Despite being distinct elements with different chemical properties, they both have an atomic mass of approximately 12 atomic mass units (amu). This similarity in atomic mass can be attributed to the way atomic masses are calculated.
Atomic mass is determined by considering the total mass of an atom's protons, neutrons, and electrons. Protons and neutrons contribute significantly to the mass, while electrons, which have a much smaller mass, are usually excluded from atomic mass calculations. Since both carbon-12 and nitrogen-14 have a similar number of protons and neutrons, their atomic masses end up being nearly identical.
Carbon-12 (12C) is the most abundant and stable isotope of carbon, with six protons and six neutrons. Its atomic mass is precisely defined as 12 amu, serving as the reference for the atomic mass unit.
On the other hand, nitrogen-14 (14N) is the most common isotope of nitrogen, featuring seven protons and seven neutrons. When atomic masses are rounded to the nearest whole number, nitrogen-14 also approximates to 14 amu, which coincidentally matches its name.
It's essential to note that while carbon-12 and nitrogen-14 share the same rounded atomic mass, they are distinct elements with different chemical properties, and this similarity is coincidental rather than indicative of a significant chemical relationship between them.
Another example of elements with identical atomic masses can be found in the case of chlorine-35 (35Cl) and sulfur-32 (32S). Chlorine-35 has 17 protons and 18 neutrons, leading to an atomic mass of approximately 35 amu. Sulfur-32, with 16 protons and 16 neutrons, also approximates to an atomic mass of 32 amu when rounded to the nearest whole number.
In conclusion, while there are instances of elements with identical atomic masses, such as carbon-12 and nitrogen-14 or chlorine-35 and sulfur-32, these similarities are a result of the way atomic masses are calculated and rounded. These elements remain distinct in terms of their chemical properties and behavior, and their shared atomic mass is a curious coincidence in the world of chemistry.