How do you calculate the molar concentration of a solution?
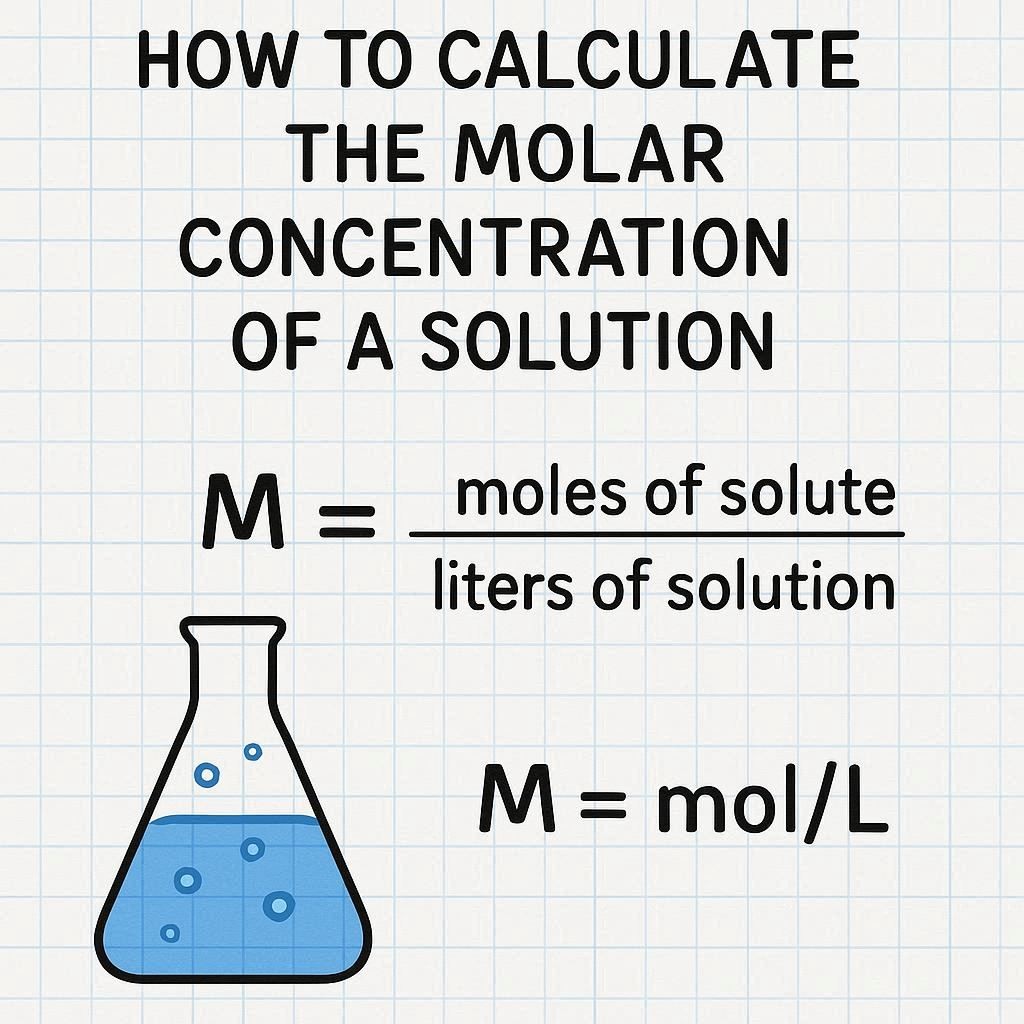
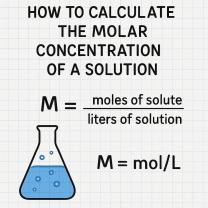
How to Calculate the Molar Concentration of a Solution
Molar concentration, also known as molarity (M), is a measure of the concentration of a solute in a solution. It tells you how many moles of a substance are dissolved in one liter of solution. Understanding how to calculate molar concentration is essential in chemistry, biology, and laboratory work.
What is Molar Concentration?
Molar concentration is expressed as:
Moles of solute: The amount of the substance dissolved, measured in moles.
Liters of solution: The total volume of the solution in liters.
Steps to Calculate Molar Concentration
1. Determine the Amount of Solute in Moles
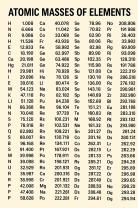
If given in grams, convert to moles using the formula:
The molar mass can be found on the periodic table by adding the atomic masses of the elements in the compound.
2. Measure the Volume of the Solution in Liters
Ensure the volume is in liters (L). Convert milliliters to liters by dividing by 1000.
3. Apply the Molarity Formula
Divide the number of moles by the volume of the solution in liters.
Example: If 0.5 moles of NaCl are dissolved in 2 liters of water:
Tips for Accurate Calculation
Always use the total solution volume, not just the volume of the solvent.
Double-check your unit conversions, especially grams to moles and milliliters to liters.
For dilute solutions, ensure proper measurement using lab glassware like volumetric flasks.
Final Thoughts
Calculating the molar concentration of a solution is a fundamental skill in chemistry. By knowing the amount of solute in moles and the volume of the solution, you can determine molarity easily using the standard formula. This calculation is essential for preparing solutions, conducting experiments, and understanding chemical reactions.
To calculate the molar concentration (or molarity) of a solution, you need to know the number of moles of solute and the total volume of the solution in liters. Molar concentration is a fundamental concept in chemistry, indicating the amount of a substance dissolved in a given volume of solution.
The video