Key Concepts
This section provides a brief overview of the fundamental principles behind atomic mass. Understanding these concepts is the first step to exploring the data for each element in the periodic table.
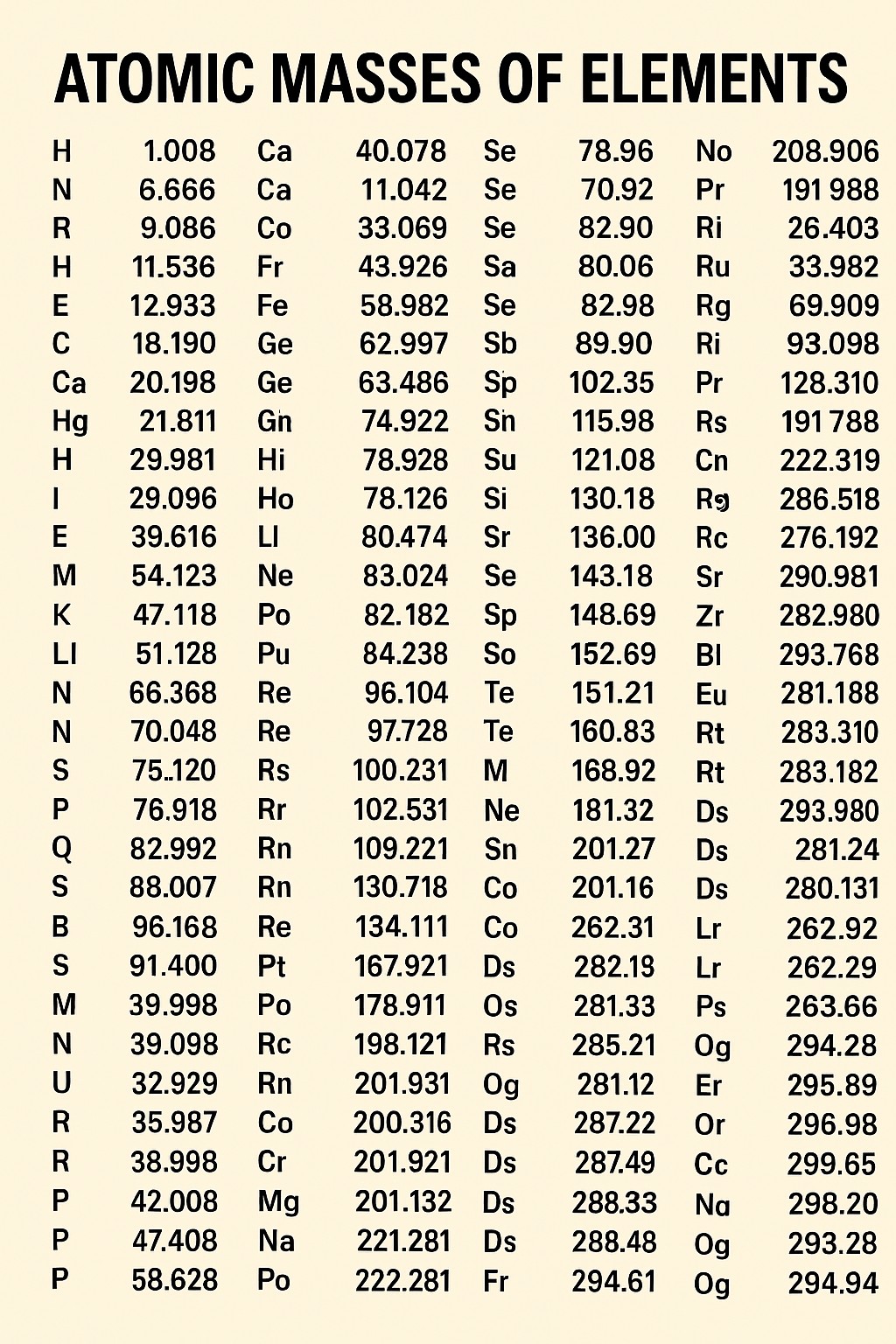
How is Atomic Mass Determined?
The atomic mass of an element is the weighted average mass of its naturally occurring isotopes. An isotope is a form of an element with a different number of neutrons. The "weighting" in the average calculation is based on the natural abundance of each isotope. For example, Carbon's atomic mass is approximately 12.011 because while most of its isotopes are Carbon-12, a small percentage are heavier isotopes like Carbon-13 and Carbon-14. This average value is determined experimentally using mass spectrometry. [Image of a mass spectrometer diagram]
What Units Are Used?
Atomic mass is typically expressed in atomic mass units (amu), also known as daltons (Da). One atomic mass unit is defined as exactly 1/12th the mass of a single, unbound atom of carbon-12 in its ground state. Therefore, the atomic mass listed on the periodic table is a dimensionless quantity relative to this standard, but it can be thought of as the average mass of an atom of that element in daltons.
Element Data Explorer
Use the tools below to search for a specific element by its name or symbol, or sort the entire list. This interactive table allows you to quickly find and compare the atomic masses of all the elements.
Visual Comparison of Common Elements
This chart displays the atomic masses of several commonly known elements. Hover over any bar to see the exact value. This visualization helps to quickly grasp the significant differences in mass between lighter and heavier elements.