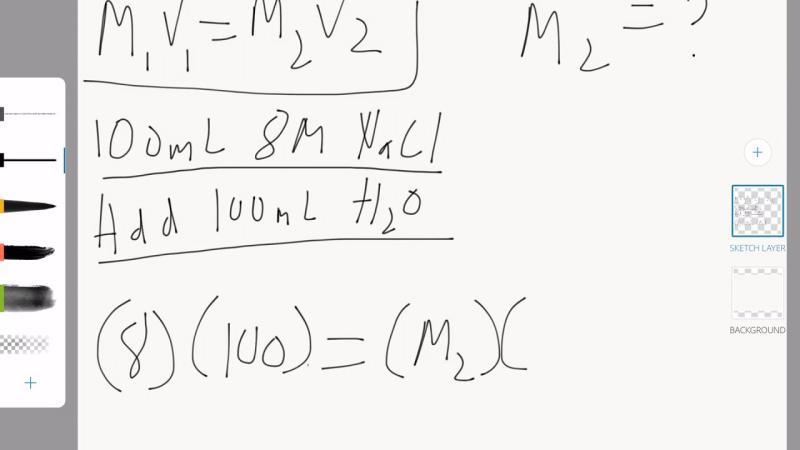What is the formula used for dilutions?
The formula for dilutions is expressed as:
where:
- is the initial concentration of the solution (before dilution),
- is the initial volume of the solution (before dilution),
- is the final concentration of the solution (after dilution), and
- is the final volume of the solution (after dilution).
This formula is commonly known as the dilution formula. It represents the conservation of moles of solute before and after dilution. In other words, the amount of solute in the solution remains constant despite changes in concentration and volume.
To use this formula, you can rearrange it to solve for any one of the four variables, depending on what information you have and what you're trying to find. Here are the rearranged forms:
These formulas are extremely useful in laboratory settings, especially in fields like chemistry and biology, where accurate preparation of solutions with specific concentrations is crucial for experiments.
Certainly, here's a comprehensive explanation of the dilution formula and its applications:
1. Dilution Formula
The dilution formula is a fundamental equation in chemistry that relates the concentration and volume of a solution before and after dilution. It is expressed as:
C₁V₁ = C₂V₂
where:
- C₁ is the initial concentration (molarity) of the solution
- V₁ is the initial volume of the solution
- C₂ is the final concentration (molarity) of the solution after dilution
- V₂ is the final volume of the solution after dilution
This formula implies that the product of the initial concentration and volume remains constant during dilution, indicating that the amount of solute (dissolved substance) remains unchanged.
2. Practical Applications of the Dilution Formula
The dilution formula finds numerous applications in various practical scenarios, including:
Preparing Solutions of Desired Concentrations: Scientists and laboratory technicians use the dilution formula to prepare solutions of specific concentrations from stock solutions of higher concentrations. This is crucial for conducting experiments, preparing reagents, and ensuring accurate measurements.
Performing Chemical Analyses: In analytical chemistry, the dilution formula plays a critical role in diluting samples to appropriate concentrations for accurate measurements and analysis. This allows for precise quantification of substances in various mixtures and materials.
Medical Applications: In medicine, the dilution formula is essential for preparing vaccines, administering medications, and conducting diagnostic tests. It ensures that patients receive the correct dosage of medications and that diagnostic tests provide reliable results.
Industrial Processes: In various industries, dilution plays a vital role in controlling chemical reactions, adjusting product concentrations, and ensuring safety protocols. It is used in manufacturing processes, pharmaceutical production, and environmental monitoring.
3. Variations of the Dilution Formula
The dilution formula can be adapted to different types of dilutions, such as serial dilutions and volumetric dilutions:
Serial Dilutions: In serial dilutions, a solution is diluted step by step, resulting in a series of solutions with decreasing concentrations. The dilution factor, which is the ratio of the final volume to the initial volume, remains constant throughout the series. This technique is used in microbiology, molecular biology, and clinical laboratory procedures.
Volumetric Dilutions: In volumetric dilutions, a specific volume of a stock solution is diluted to a desired final volume using a diluent (usually water). The dilution factor is calculated accordingly. This technique is commonly used in analytical chemistry and pharmaceutical preparations.
Regardless of the dilution type, the underlying principle of the dilution formula remains the same: the amount of solute remains constant during dilution.