What temperature is water the most dense?
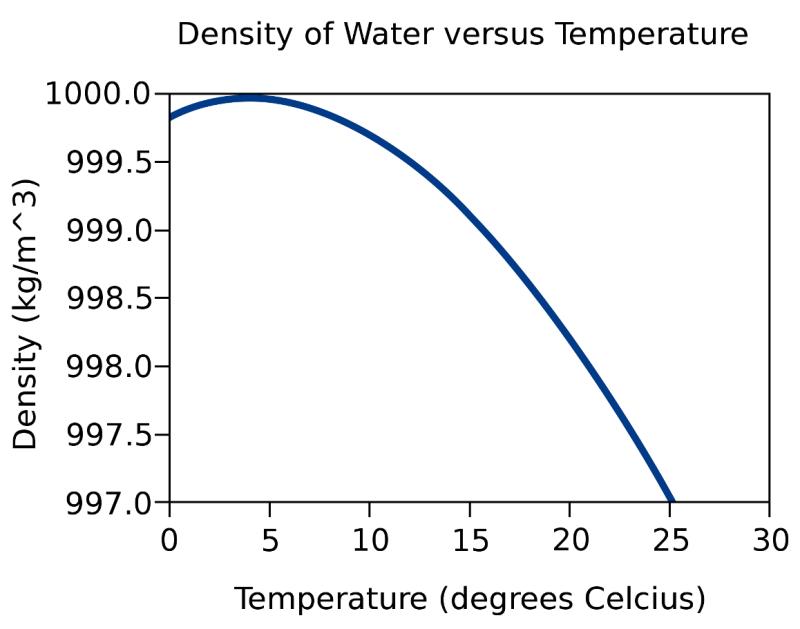
Water is at its maximum density at around 4 degrees Celsius (39.2 degrees Fahrenheit). This means that at this temperature, water is at its highest density, and as the temperature either decreases or increases from this point, its density decreases.
Interestingly, when water freezes and turns into ice, it expands rather than contracts, which is contrary to what happens with most substances. This expansion is why ice floats on water. This unique behavior is due to the way water molecules arrange themselves into a crystalline structure when freezing, creating a pattern with more space between molecules compared to the densely packed arrangement in liquid water.
Water's Density Dance with Temperature:
Water, that seemingly simple molecule, has a surprising quirk when it comes to density and temperature. Let's dive in:
1. Peak Performance: The Densest Water
Water reaches its maximum density at around 4°C (39°F). This might seem counterintuitive, as we expect things to shrink as they get colder. But for water, hydrogen bonding, the attractive force between water molecules, plays a crucial role.
At temperatures above 4°C, water molecules are zipping around with more thermal energy, disrupting the organized hydrogen bonding network. This makes the water expand, and its density decreases.
2. The Density Rollercoaster: Up and Down with Temperature
As water cools below 4°C, however, the hydrogen bonds become stronger and more ordered. This pulls the molecules closer, packing them into a denser arrangement. This trend continues until 4°C, where water is at its most tightly packed and densest.
However, the story doesn't end there. As water cools further below 4°C, towards its freezing point (0°C), it starts forming ice crystals. Ice crystals have a more open, structured arrangement compared to liquid water. This means that even though the individual molecules are getting colder and potentially closer, the overall structure of ice is less dense than liquid water at 4°C.
Therefore, water density starts to decrease again as it approaches freezing. This is why ice floats in water, a vital property for life on Earth as it allows bodies of water to freeze from the top down, leaving liquid water below for organisms to survive.
In a nutshell:
- Water is densest at around 4°C (39°F).
- Above 4°C, increasing thermal energy disrupts hydrogen bonds, leading to expansion and lower density.
- Below 4°C, stronger hydrogen bonds pack molecules tightly, increasing density until freezing.
- Ice formation creates a less dense structure, causing density to decrease again towards 0°C.
Remember, water's density dance with temperature is a fascinating example of how microscopic interactions can have a macro-scale impact. So next time you sip on a cold glass of water, appreciate the intricate interplay of temperature and density happening within that seemingly simple liquid.