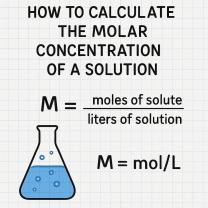
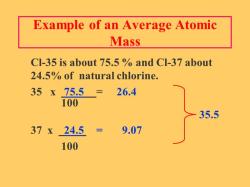
What are the common chemical reactions?
Chemical reactions are fundamental processes in chemistry where substances undergo transformations, resulting in the formation of new substances. There are several common types of chemical reactions, each with its unique characteristics and outcomes. Here are some of the most common chemical reactions:
Combination Reactions (Synthesis Reactions):
- In a combination reaction, two or more substances combine to form a single, more complex compound. For example:
- 2H2 (g) + O2 (g) → 2H2O (g) (formation of water)
- C (s) + O2 (g) → CO2 (g) (formation of carbon dioxide)
- In a combination reaction, two or more substances combine to form a single, more complex compound. For example:
Decomposition Reactions:
- Decomposition reactions involve the breakdown of a single compound into two or more simpler substances. Examples include:
- 2H2O (l) → 2H2 (g) + O2 (g) (decomposition of water)
- 2KClO3 (s) → 2KCl (s) + 3O2 (g) (decomposition of potassium chlorate)
- Decomposition reactions involve the breakdown of a single compound into two or more simpler substances. Examples include:
Single Replacement Reactions:
- In single replacement reactions, one element replaces another in a compound. The general form is:
- A + BC → AC + B
- Example: Zn (s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g)
- In single replacement reactions, one element replaces another in a compound. The general form is:
Double Replacement Reactions (Metathesis Reactions):
- Double replacement reactions involve the exchange of ions between two compounds. The general form is:
- AB + CD → AD + CB
- Example: AgNO3 (aq) + NaCl (aq) → AgCl (s) + NaNO3 (aq)
- Double replacement reactions involve the exchange of ions between two compounds. The general form is:
Acid-Base Reactions:
- Acid-base reactions involve the transfer of protons (H+ ions) between an acid and a base. These reactions often produce water and a salt. Example:
- HCl (aq) + NaOH (aq) → H2O (l) + NaCl (aq)
- Acid-base reactions involve the transfer of protons (H+ ions) between an acid and a base. These reactions often produce water and a salt. Example:
Redox Reactions (Oxidation-Reduction Reactions):
- Redox reactions involve the transfer of electrons between reactants. Oxidation is the loss of electrons, while reduction is the gain of electrons. Combustion reactions, like the burning of fuels, are common redox reactions:
- 2C4H10 (g) + 13O2 (g) → 8CO2 (g) + 10H2O (g)
- Redox reactions involve the transfer of electrons between reactants. Oxidation is the loss of electrons, while reduction is the gain of electrons. Combustion reactions, like the burning of fuels, are common redox reactions:
Precipitation Reactions:
- Precipitation reactions involve the formation of an insoluble solid (precipitate) when two solutions are mixed. Example:
- Pb(NO3)2 (aq) + 2KI (aq) → PbI2 (s) + 2KNO3 (aq)
- Precipitation reactions involve the formation of an insoluble solid (precipitate) when two solutions are mixed. Example:
Neutralization Reactions:
- Neutralization reactions are a specific type of acid-base reaction where an acid and a base combine to form water and a salt. For example:
- H2SO4 (aq) + 2NaOH (aq) → 2H2O (l) + Na2SO4 (aq)
- Neutralization reactions are a specific type of acid-base reaction where an acid and a base combine to form water and a salt. For example:
Hydrolysis Reactions:
- Hydrolysis reactions involve the breakdown of a compound by water, often resulting in the formation of an acid and a base. Example:
- Na2CO3 (aq) + H2O (l) → NaHCO3 (aq) + NaOH (aq)
- Hydrolysis reactions involve the breakdown of a compound by water, often resulting in the formation of an acid and a base. Example:
Photosynthesis:
- This biological reaction takes place in plants and some microorganisms, where carbon dioxide and water are converted into glucose and oxygen in the presence of light and chlorophyll.
These common chemical reactions form the basis for understanding and predicting chemical changes in various fields, from chemistry and biology to industry and everyday life. Each type of reaction has specific characteristics and principles that govern its occurrence.
Exploring Common Chemical Reactions and Their Types
Chemical reactions are the processes by which atoms and molecules rearrange to form new substances. They are essential for life and occur all around us, from the food we eat to the air we breathe.
There are many different types of chemical reactions, but some of the most common include:
- Combination reactions: Two or more substances combine to form a new substance. For example:
H2 + O2 → H2O
- Decomposition reactions: A single substance breaks down into two or more simpler substances. For example:
2H2O → 2H2 + O2
- Single-displacement reactions: One element displaces another element from a compound. For example:
Zn + CuSO4 → ZnSO4 + Cu
- Double-displacement reactions: Two compounds exchange ions to form two new compounds. For example:
NaCl + AgNO3 → NaNO3 + AgCl
The Role of Chemical Reactions in Everyday Life
Chemical reactions play a role in many aspects of our everyday lives. For example:
- Digestion: The food we eat breaks down into smaller molecules through chemical reactions.
- Respiration: We breathe in oxygen and breathe out carbon dioxide through chemical reactions.
- Combustion: When we burn fuel, such as gasoline or wood, the fuel reacts with oxygen to release heat and light.
- Cooking: Many cooking processes, such as baking and frying, involve chemical reactions.
- Cleaning: Many cleaning products contain chemicals that react with dirt and grime to remove them.
Chemical reactions are also used to produce a wide variety of products, including:
- Medications
- Plastics
- Fertilizers
- Paints
- Batteries
- Fuel
Basic Principles of Chemical Reaction Kinetics
Chemical reaction kinetics is the study of the rates of chemical reactions. It is concerned with the factors that affect the speed of a reaction and the mechanisms by which reactions occur.
Some of the factors that affect the rate of a chemical reaction include:
- Temperature: Higher temperatures generally increase the rate of a reaction.
- Concentration: The higher the concentration of the reactants, the faster the reaction will occur.
- Surface area: The greater the surface area of the reactants, the faster the reaction will occur.
- Presence of a catalyst: A catalyst is a substance that increases the rate of a reaction without being consumed in the reaction.
Balancing Chemical Equations: A Fundamental Skill in Chemistry
A chemical equation is a symbolic representation of a chemical reaction. It shows the reactants, products, and the stoichiometric coefficients of the reaction.
Balancing a chemical equation involves adjusting the coefficients of the reactants and products so that the number of atoms of each element is the same on both sides of the equation.
Balancing chemical equations is an important fundamental skill in chemistry because it allows chemists to calculate the amount of reactants and products needed for a reaction, as well as the amount of energy released or absorbed during the reaction.
Real-World Applications of Chemical Reactions
Chemical reactions are used in a wide variety of real-world applications, including:
- Energy production: Chemical reactions are used to generate electricity from fossil fuels, nuclear power, and renewable energy sources.
- Food and agriculture: Chemical reactions are used to produce fertilizers, pesticides, and herbicides. They are also used to process food and beverages.
- Materials science: Chemical reactions are used to produce a wide variety of materials, including plastics, metals, and ceramics.
- Medicine: Chemical reactions are used to produce pharmaceuticals and other medical products.
- Environmental science: Chemical reactions are used to clean up pollution and develop new environmental technologies.
Chemical reactions are essential for life and play a vital role in many aspects of our modern world. By understanding the basic principles of chemical reactions, we can better appreciate the world around us and develop new technologies to improve our lives.