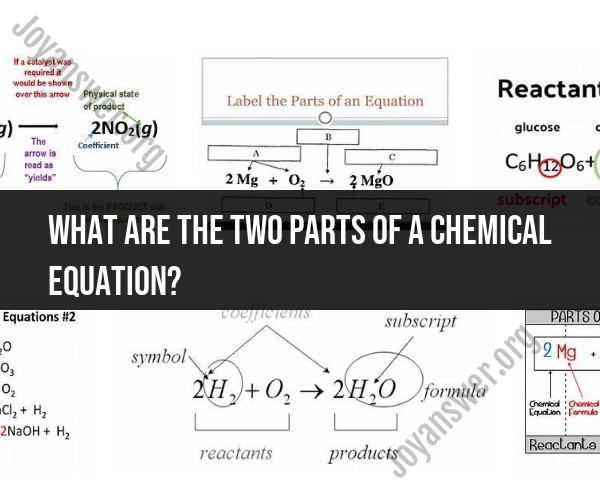
What are the two parts of a chemical equation?
A chemical equation consists of two main parts:
Reactants: These are the substances that are present at the beginning of a chemical reaction. Reactants are the chemicals that undergo a change during the reaction. They are written on the left side of the chemical equation and are often separated by plus signs (+).
Products: These are the substances that are formed as a result of the chemical reaction. Products are the chemicals that result from the rearrangement of atoms in the reactants. They are written on the right side of the chemical equation and are also separated by plus signs (+).
In a balanced chemical equation, the number of atoms of each element on the left side (reactants) must be equal to the number of atoms of the same element on the right side (products). This conservation of atoms is a fundamental principle in chemistry. The equation serves as a symbolic representation of a chemical reaction, showing what substances are involved and what changes occur as a result of the reaction.