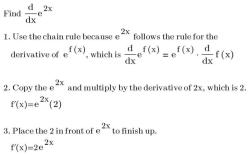How do you convert percentage to mass?
Converting a percentage to mass involves calculating the actual amount of a substance or component based on its percentage by mass in a given sample or mixture. To do this, you'll need to know the total mass of the sample or mixture and the percentage by mass of the specific substance you want to find. You can use the following formula:
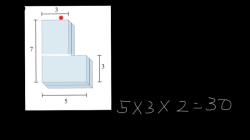
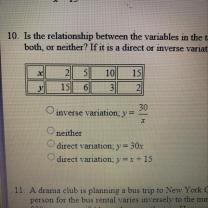
Mass = (Percentage / 100) × Total Mass
Where:
- Mass is the mass of the specific substance you want to find.
- Percentage is the percentage by mass of the substance in the sample.
- Total Mass is the total mass of the sample or mixture.
Here's a step-by-step example:
Let's say you have a 500-gram mixture of sand, and you want to find out how much salt is in the mixture. You've determined that the salt content is 10% by mass.
Identify the values:
- Percentage (Salt content) = 10%
- Total Mass (Mixture) = 500 grams
Use the formula:
- Mass (Salt) = (10 / 100) × 500
- Mass (Salt) = (0.10) × 500
Calculate:
- Mass (Salt) = 50 grams
So, in your 500-gram mixture, there are 50 grams of salt.
This formula can be used to convert any percentage by mass into an actual mass value, as long as you know the total mass of the sample or mixture and the percentage of interest.
Percentage to Mass Conversion: Mastering the Math Behind It
To convert percentage to mass, you need to know the total mass of the mixture or solution. Then, multiply the percentage by the total mass to find the mass of the desired component.
For example, let's say you have a 100-gram solution of salt water that is 10% salt. To find the mass of salt in the solution, you would multiply 10% by 100 grams. This gives you 10 grams of salt.
Here is the formula for converting percentage to mass:
Mass of component = Percentage * Total mass
Chemistry Calculations: Converting Percentage to Mass
In chemistry, percentage to mass conversion is often used to find the mass of a solute in a solution. To do this, you need to know the concentration of the solute in the solution, as well as the volume of the solution.
For example, let's say you have a 100-mL solution of hydrochloric acid that is 1 M. To find the mass of hydrochloric acid in the solution, you need to know the molar mass of hydrochloric acid, which is 36.46 grams per mole.
Then, multiply the concentration by the volume to find the moles of hydrochloric acid in the solution. This gives you 1 mole of hydrochloric acid.
Finally, multiply the moles of hydrochloric acid by the molar mass to find the mass of hydrochloric acid in the solution. This gives you 36.46 grams of hydrochloric acid.
Here is the formula for converting percentage to mass in chemistry:
Mass of solute = Concentration * Volume * Molar mass
Mass and Percentage: How to Switch Between the Two in Chemistry
To switch between mass and percentage in chemistry, you can use the following formulas:
Percentage = (Mass of component / Total mass) * 100%
Mass of component = (Percentage / 100%) * Total mass
For example, let's say you have a 100-gram mixture of salt and sugar that is 50% salt. To find the mass of sugar in the mixture, you would use the following formula:
Mass of sugar = (50% / 100%) * 100 grams = 50 grams
Therefore, the mass of sugar in the mixture is 50 grams.