How can I calculate percent concentration by mass?
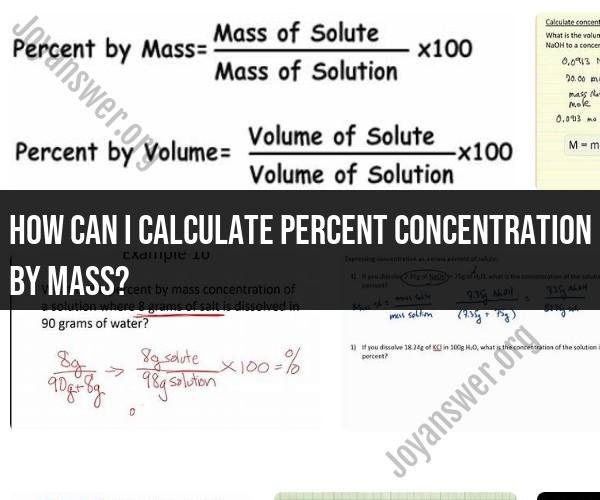
To calculate percent concentration by mass, you'll need to determine the mass of the solute (the substance being dissolved) and the total mass of the solution (the solute and solvent combined). The formula for calculating percent concentration by mass is:
Here are the steps to calculate percent concentration by mass:
Step 1: Determine the Mass of the SoluteWeigh the mass of the solute using a balance or scale. The mass should be measured in the same unit (e.g., grams) as the total mass of the solution.
Step 2: Determine the Total Mass of the SolutionWeigh the total mass of the solution, which includes both the solute and the solvent. Make sure to use the same unit as in step 1.
Step 3: Calculate the Percent Concentration by MassUse the formula mentioned earlier to calculate the percent concentration by mass. Divide the mass of the solute by the total mass of the solution and then multiply by 100 to express the result as a percentage.
Here's an example:
Suppose you want to calculate the percent concentration by mass of salt (sodium chloride, NaCl) in a solution. You weigh 2 grams of salt (the solute) and then dissolve it in 98 grams of water (the solvent) to make a total solution mass of 100 grams.
So, the percent concentration by mass of salt in the solution is 2%.
Keep in mind that percent concentration by mass is commonly used in chemistry to express the concentration of solutes in solutions. It's a simple and widely used method to convey the relative amount of a substance in a mixture.
Calculating Percent Concentration by Mass: Step-by-Step Guide
To calculate the percent concentration by mass of a solution, follow these steps:
- Determine the mass of the solute. The solute is the substance that is dissolved in the solution.
- Determine the mass of the solution. The solution is the mixture of the solute and the solvent.
- Divide the mass of the solute by the mass of the solution and multiply by 100%. This will give you the percent concentration by mass of the solution.
Example:
A solution contains 10 grams of salt dissolved in 100 grams of water. What is the percent concentration by mass of the solution?
Percent concentration by mass = (mass of solute / mass of solution) * 100%
Percent concentration by mass = (10 grams / 110 grams) * 100%
Percent concentration by mass = 9.09%
Therefore, the percent concentration by mass of the solution is 9.09%.
Understanding Mass Percent: Definition and Significance
Mass percent is a unit of concentration that expresses the mass of a solute in a solution as a percentage of the total mass of the solution. It is a common way to express the concentration of solutions in chemistry, especially for solutions of solids dissolved in liquids.
Mass percent is significant because it is a convenient and easy-to-understand way to express the concentration of a solution. It is also a unit of concentration that is widely used in industry and manufacturing.
Formulas and Equations for Determining Percent Concentration
The following formula is used to determine the percent concentration by mass of a solution:
Percent concentration by mass = (mass of solute / mass of solution) * 100%
where:
- Mass of solute is the mass of the substance that is dissolved in the solution.
- Mass of solution is the mass of the mixture of the solute and the solvent.
The following formula is used to determine the mass of solute in a solution given the percent concentration by mass and the mass of solution:
Mass of solute = (percent concentration by mass / 100%) * mass of solution
Real-World Applications and Problem-Solving with Percent Mass
Percent mass is used in a variety of real-world applications, including:
- Chemistry: Percent mass is used to express the concentration of solutions in chemistry, especially for solutions of solids dissolved in liquids.
- Industry: Percent mass is used to express the concentration of solutions in industry, such as in the production of food, beverages, and pharmaceuticals.
- Manufacturing: Percent mass is used to express the concentration of solutions in manufacturing, such as in the production of paints, coatings, and adhesives.
Here is an example of a problem that can be solved using percent mass:
A solution of salt water has a percent concentration by mass of 5%. What is the mass of salt in 100 grams of the solution?
Mass of salt = (percent concentration by mass / 100%) * mass of solution
Mass of salt = (5% / 100%) * 100 grams
Mass of salt = 5 grams
Therefore, there are 5 grams of salt in 100 grams of the solution.
Accuracy and Precision in Mass Percent Calculations
The accuracy and precision of mass percent calculations depend on the accuracy and precision of the measurements of the mass of the solute and the mass of the solution.
If the mass of the solute and the mass of the solution are measured accurately and precisely, then the mass percent calculation will also be accurate and precise. However, if the mass of the solute or the mass of the solution is not measured accurately and precisely, then the mass percent calculation may not be accurate and precise.
To improve the accuracy and precision of mass percent calculations, it is important to use a calibrated balance to measure the mass of the solute and the mass of the solution. It is also important to follow the instructions for using the balance carefully.