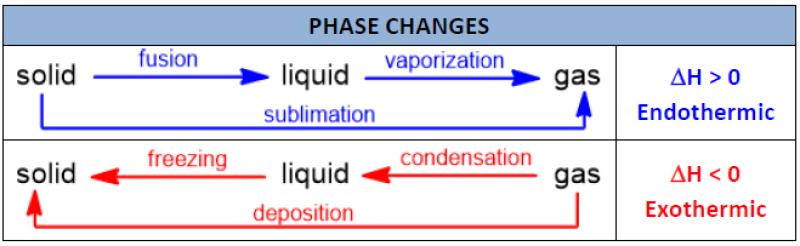
What are the six different phase changes?
Phase changes, also known as state changes, refer to the transitions between the different states of matter—solid, liquid, and gas. There are six main phase changes, and they involve the transfer of energy, typically in the form of heat. Here are the six different phase changes:
Melting (Solid to Liquid):
- Description: The process in which a solid substance absorbs enough heat energy to overcome the forces holding its particles together. This results in the substance transitioning from a solid to a liquid state.
- Example: Ice melting into water.
Freezing (Liquid to Solid):
- Description: The process in which a liquid substance loses enough heat energy to allow its particles to come together and form a solid structure.
- Example: Water freezing into ice.
Vaporization (Liquid to Gas):
- Description: The process in which a liquid substance absorbs enough heat energy to break the bonds between its particles, allowing them to move freely and form a gas.
- Example: Boiling water to produce steam.
Condensation (Gas to Liquid):
- Description: The process in which a gaseous substance loses enough heat energy to cause its particles to come together and form a liquid.
- Example: Steam condensing back into liquid water on a cold surface.
Sublimation (Solid to Gas):
- Description: The process in which a solid substance transforms directly into a gas without passing through the liquid phase. This occurs when the substance absorbs enough heat energy.
- Example: Dry ice (solid carbon dioxide) sublimating into carbon dioxide gas.
Deposition (Gas to Solid):
- Description: The process in which a gas transforms directly into a solid without passing through the liquid phase. This occurs when the gas loses enough heat energy.
- Example: Water vapor in the air turning directly into frost on a cold surface.
These phase changes are fundamental concepts in the study of thermodynamics and the behavior of matter. They occur in response to changes in temperature and pressure and play a crucial role in various natural processes and industrial applications. Understanding these transitions helps explain the properties and behaviors of different materials under different conditions.
The six different phase changes in matter are:
- Melting: The change from solid to liquid phase.
- Freezing: The change from liquid to solid phase.
- Evaporation: The change from liquid to gas phase.
- Condensation: The change from gas to liquid phase.
- Sublimation: The change from solid to gas phase without passing through the liquid phase.
- Deposition: The change from gas to solid phase without passing through the liquid phase.
Temperature and pressure influence phase changes in the following ways:
- Temperature: Increasing the temperature of a substance can cause it to melt, evaporate, or sublime. Decreasing the temperature of a substance can cause it to freeze, condense, or deposit.
- Pressure: Increasing the pressure on a substance can cause it to melt or freeze. Decreasing the pressure on a substance can cause it to evaporate or sublime.
Here are some examples of substances undergoing phase changes:
- Melting: Ice melting into water
- Freezing: Water freezing into ice
- Evaporation: Water evaporating into steam
- Condensation: Steam condensing into water droplets
- Sublimation: Dry ice sublimating into carbon dioxide gas
- Deposition: Frost forming on a window
Phase changes are an important part of our everyday lives. For example, we use the phase change of water from liquid to gas to cook food and to generate electricity. We also use the phase change of water from gas to liquid to produce rain and snow.