What is the osmolality of IV fluid?
The osmolality of an intravenous (IV) fluid refers to the concentration of solute particles (such as electrolytes and other dissolved substances) in the fluid. It measures the number of solute particles per kilogram of solvent (usually water). Osmolality is expressed in units of milliosmoles per kilogram (mOsm/kg) or sometimes as osmoles per kilogram (Osm/kg).
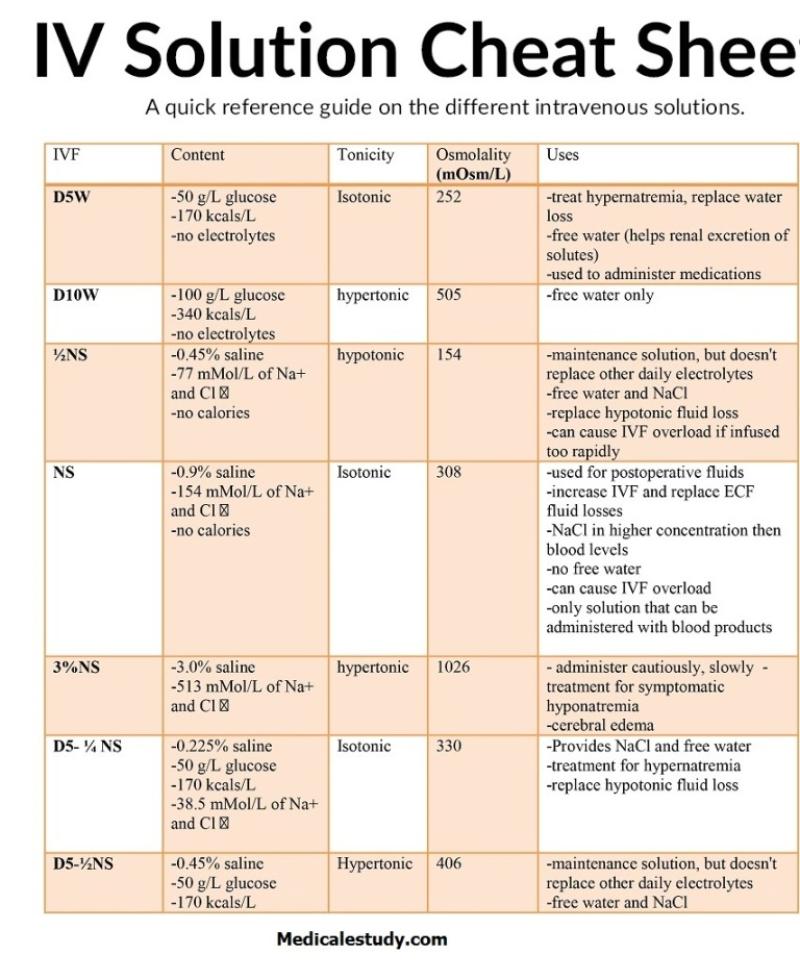
IV fluids are classified into different types based on their osmolality, and the choice of IV fluid is important in various medical scenarios. The primary categories of IV fluids in terms of osmolality include:
Hypotonic IV Fluids: These fluids have an osmolality lower than that of normal body fluids. They contain fewer solute particles than the body's cells and extracellular fluid. Hypotonic IV fluids are often used to provide hydration and to replace lost fluids. They may include solutions like 0.45% saline (half-normal saline).
Isotonic IV Fluids: Isotonic IV fluids have an osmolality similar to that of normal body fluids. They have a balanced concentration of solute particles, and they are often used to maintain fluid balance and electrolyte levels. Common examples of isotonic fluids include 0.9% saline (normal saline) and lactated Ringer's solution.
Hypertonic IV Fluids: Hypertonic IV fluids have a higher osmolality than that of normal body fluids. They contain a higher concentration of solute particles. Hypertonic fluids are used in specific medical situations to draw fluids from the body's cells and increase extracellular fluid volume. They may include solutions like 3% saline or 5% dextrose in normal saline.
The choice of IV fluid and its osmolality is determined by the patient's condition, needs, and clinical goals. For example, isotonic fluids are commonly used for general hydration and to maintain blood pressure, while hypertonic fluids may be used to treat certain medical conditions or to draw fluids into the circulatory system in cases of severe dehydration.
It's crucial for healthcare professionals to understand osmolality and select the appropriate IV fluid to address the specific needs of the patient, ensuring that the fluid is administered safely and effectively. The wrong choice of IV fluid can lead to complications, including fluid and electrolyte imbalances.
Understanding Osmolality and Its Role in IV Fluids
Osmolality is a measure of the concentration of solute particles in a solution. In the context of intravenous (IV) fluids, osmolality plays a crucial role in determining the fluid's ability to move water across cell membranes.
2. Measuring Osmolality in Intravenous Fluids
Osmolality is typically measured in milliosmoles per kilogram (mOsm/kg). There are two main methods for measuring osmolality:
Osmometers: These devices directly measure the number of solute particles in a solution.
Freezing point depression: This method measures the change in freezing point of a solution compared to pure water, which is proportional to the concentration of solute particles.
Osmolality Guidelines for Different Types of IV Fluids
Different types of IV fluids have different osmolalities, each serving a specific purpose:
Isotonic fluids: These fluids have an osmolality similar to that of blood plasma, typically around 300 mOsm/kg. They are used to maintain fluid balance and electrolyte levels without causing fluid shifts between cells and the extracellular space.
Hypotonic fluids: These fluids have an osmolality lower than that of blood plasma, typically around 250 mOsm/kg or less. They are used to replace fluids lost due to dehydration or to promote diuresis (increased urine output).
Hypertonic fluids: These fluids have an osmolality higher than that of blood plasma, typically around 325 mOsm/kg or more. They are used to draw fluid from tissues into the vascular compartment, such as in cases of severe burns or edema.
Clinical Significance of Osmolality in IV Fluid Selection
Choosing the appropriate osmolality of IV fluids is crucial for maintaining fluid balance, preventing electrolyte imbalances, and avoiding potential complications. Factors to consider when selecting IV fluids include:
Patient's clinical status: The patient's medical condition, fluid balance status, and presence of any underlying diseases will influence the choice of osmolality.
Nature of the fluid loss: The cause of fluid loss, such as dehydration, hemorrhage, or burns, will determine the type of IV fluid required.
Rate of fluid administration: The rate at which IV fluids are administered can affect the movement of water between cells and the extracellular space.
Potential Complications of IV Fluids with Inappropriate Osmolality
Using IV fluids with inappropriate osmolality can lead to various complications:
Hypotonic fluids: Excessive use of hypotonic fluids can cause cell swelling, potentially leading to hemolysis (destruction of red blood cells) and electrolyte imbalances.
Hypertonic fluids: Excessive use of hypertonic fluids can cause fluid shifts from cells to the extracellular space, leading to dehydration of cells and potential organ damage.
Electrolyte imbalances: Inappropriate osmolality of IV fluids can disrupt electrolyte balance, leading to symptoms such as muscle cramps, weakness, and cardiac arrhythmias.
In conclusion, osmolality is a critical factor in IV fluid therapy, influencing fluid distribution, electrolyte balance, and potential complications. Healthcare professionals carefully evaluate the patient's condition and clinical needs to select the appropriate osmolality of IV fluids to ensure optimal patient care.