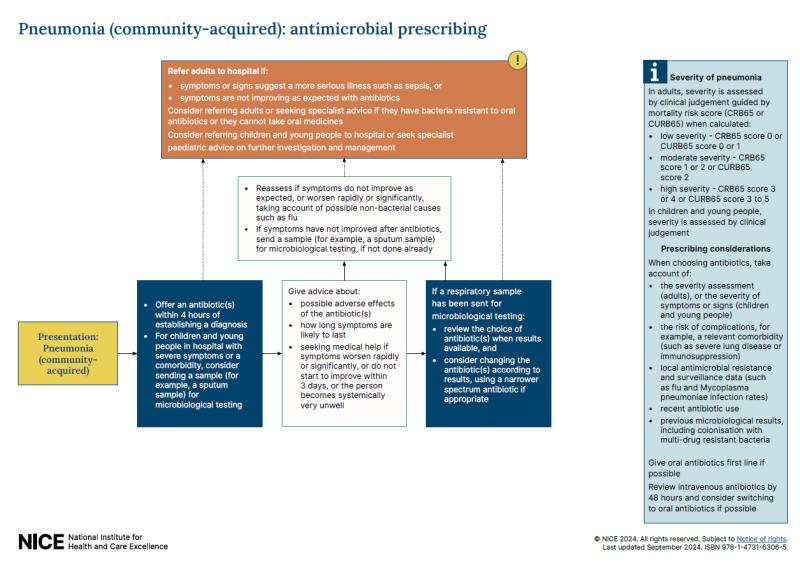
What is the difference between NICE guidelines and related NICE guidance?
The key to understanding the difference between "NICE guidelines" and "related NICE guidance" is to recognize that "NICE guidance" is a broad term that encompasses many different types of publications, while "NICE guidelines" are a specific type of guidance.
Here's a breakdown of the distinction:
NICE Guidance (the umbrella term):
This refers to all of the evidence-based recommendations and advice produced by the National Institute for Health and Care Excellence (NICE).
Clinical Guidelines: Recommendations on the appropriate treatment and care for people with specific diseases and conditions.
These are a major component of NICE's work. Technology Appraisals: Guidance on the use of new and existing medicines, treatments, and procedures.
This often focuses on whether a new technology is clinically effective and cost-effective for the NHS. Medical Technologies Guidance: Evaluation of innovative medical devices and diagnostic tools.
Interventional Procedures Guidance: Recommendations on whether surgical and other interventional procedures are safe and effective enough for use in the NHS.
Public Health Guidance: Recommendations on promoting good health and preventing ill health.
Quality Standards: A set of concise, specific statements that define high-quality care for a particular topic.
NICE Guidelines (a specific type of guidance):
This refers specifically to the evidence-based recommendations on how healthcare professionals should care for people with particular conditions or diseases.
Understanding NICE Guidelines and Guidance
The UK’s NICE (National Institute for Health and Care Excellence) issues advice to help improve health and social care. This includes both NICE guidance (the overall term) and specific NICE guidelines (a type of guidance). In simple terms:
NICE guidance is the umbrella category for all of NICE’s recommendations on health and care. It includes many forms of advice, such as technology appraisals (for drugs), procedures, and morenice.org.uk.
NICE guidelines are one kind of guidance – evidence-based recommendations specifically about clinical, social care, public health or medicines topicsnice.org.uk. These guidelines tell healthcare workers the best ways to prevent illness, promote health, and improve care.
In short: guidance means any recommendation NICE makes, while guidelines are the formal advice documents on treating and managing conditionsnice.org.uk.
How Are NICE Guidelines Developed?
NICE uses a rigorous, transparent process to create guidelines. Key steps include:
Topic selection: Government bodies (e.g. NHS England, the Department of Health) refer topics to NICE. These might be diseases, treatments, public health issues, or social care questionsnice.org.uk.
Scoping the guideline: NICE staff draft a scope document explaining the need for the guideline and what it will cover (and what it won’t). Stakeholders (doctors, patients, charities, etc.) review this draft scope and suggest changesnice.org.uk.
Evidence review: An independent guideline committee is formed (usually doctors, nurses, other professionals, patients or carers). This committee agrees on clinical questions and systematically searches medical literature. They compile and appraise all relevant research and data. The committee looks at things like how strong the evidence is and how much things costnice.org.uknice.org.uk.
Draft recommendations: Based on the evidence, the committee writes draft recommendations (advice on treatment or care). This draft is posted for public consultation so that experts, organizations and the public can commentnice.org.uk.
Revision: The committee reviews the feedback from the consultation. They make any needed changes and check the quality of the guideline.nice.org.uk
Approval and publication: A senior NICE team (the Guidance Executive) gives final approval, and the guideline is published on the NICE websitenice.org.uk. NICE then works with the NHS and others to share the new guideline.
Updating: After publication, the guideline is regularly reviewed. If new important evidence emerges, NICE updates the recommendationsnice.org.uk.
Throughout, NICE emphasizes stakeholder involvement. Its official materials note that guidelines are “based on the best available evidence” and are “put together by experts, people using services, carers and the public”nice.org.uk. In practice, that means patients and carers help shape the questions and priorities, and a multidisciplinary committee considers both research and real-world views when writing each guidelinenice.org.uknice.org.uk.
Types of guidance NICE provides
NICE issues many kinds of guidance, each with a specific purpose. These include:
Clinical, public health and social care guidelines: Broad recommendations on preventing and treating diseases or improving care. For example, a clinical guideline might cover diabetes management or asthma care.
Medicines practice guidelines: Advice on prescribing and using medicines safely.
Technology Appraisals (TAs): These are detailed reviews of new drugs or treatments. NICE evaluates their clinical and cost effectiveness. Importantly, if a treatment is recommended in a TA, the NHS must fund itnice.org.uk.
Medical Technologies guidance: Advice on innovative medical devices or diagnostic tests that improve patient care.
Diagnostics guidance: Reviews of new tests or scans to diagnose conditions.
Interventional Procedures guidance: Guidance on medical or surgical procedures (e.g. laser eye surgery) to decide if they are safe and effective enough for NHS use.
Highly Specialised Technologies guidance: Similar to TAs but for very rare diseases (specialist medicines or therapies used in only a few patients).
Evidence Summaries: Quick overviews of the latest evidence for new medicines or treatments (often used for local decision-making).
Each type of guidance focuses on evidence-based best practice. For example, technology appraisals look at new medicines and decide whether they work well enough to use in the NHS (and if so, NHS must make them available)nice.org.uk. Interventional procedure guidance might simply say whether a surgery is proven to be safe. This range of guidance helps cover everything from day-to-day clinical care to big policy decisions.
How Do Healthcare Professionals Use These Guidelines?
Doctors, nurses and other professionals use NICE guidelines as a trusted source of advice. In practice, they:
Inform treatment decisions: When a doctor is deciding how to treat a patient (e.g. which drug or therapy to use), they often check the relevant NICE guideline. NICE explains what works best for patients. In NICE’s words, guidelines help professionals “make informed decisions on the appropriate treatment and care”rnid.org.uk. For example, a guideline might say “first try medicine A, and if that doesn’t work consider medicine B.”
Guide prevention and health promotion: Many guidelines include advice on preventing illness. For instance, public health guidelines cover things like stopping smoking or managing diet, and social care guidelines might cover helping at-home care for the elderly. NICE itself notes that its guidelines help professionals “prevent ill health [and] promote good health”nice.org.uk.
Support patient discussions (shared decisions): NICE guidelines often highlight where patient preferences matter. They explicitly say their guidance “support[s] shared decision making between healthcare professionals and people receiving care”nice.org.uk. In other words, doctors use NICE advice to explain options and risks, helping patients choose what’s best for them.
Plan care and resources: Clinical teams and NHS managers use guidelines to design care pathways and checklists. For example, a clinic might audit its asthma patients against the NICE recommendations to see if they are all receiving the right tests and medicines. NICE notes that following guidelines can help target healthcare resources “to significantly improve patient outcomes”rnid.org.uk.
Training and standards: Guidelines also serve as training material for medical staff and as a benchmark for quality. Hospitals and clinics may require that their staff follow the latest NICE guidelines as part of protocols or quality improvement. (However, it’s worth noting that, except for technology appraisals, NICE guidance is technically advisory. As RNID explains, doctors “are not legally obliged to follow NICE guidelines, [but] are expected to take them into full account”rnid.org.uk.)
In sum, NICE guidance shapes everyday practice: it tells health professionals which tests to run, which treatments to choose, and how to organize services for better care.
Why Are Clear Distinctions Important in Medical Guidance?
It’s crucial that everyone understands what kind of NICE advice they’re using, because different documents have different implications. Clear terms prevent confusion and ensure correct use:
Mandatory vs. advisory: For example, a Technology Appraisal is binding – the NHS must fund medicines it recommendsnice.org.uk. In contrast, a clinical guideline is advisory: it’s expert-backed advice but not legally enforceable. If professionals mix these up, they could misinterpret a recommendation. Knowing the difference helps healthcare organizations implement guidance correctly.
Understanding scope and use: NICE itself emphasizes the terminology to avoid mix-ups. The NICE style guide explicitly says to reserve “guidance” for all recommendation documents and “guidelines” for the clinical/social care topicsnice.org.uk. This discipline in language means a clear reader knows exactly what kind of advice they’re looking at.
Patient communication and trust: Patients and carers also benefit when guidance is clearly labeled. They can see whether a suggestion is a well-established guideline or a newer piece of advice. Clear language builds trust and makes sure patients and professionals alike know which guidance to rely on in each situation.
In short, precise use of terms and categories ensures that NICE’s recommendations are interpreted correctly and used as intended – whether as mandatory instructions (in the case of approved treatments) or as best-practice advice for care.
Sources: Explanations are based on NICE’s official publications and guidelinesnice.org.uknice.org which describe the development process and use of NICE guidance in the UK. Each citation corresponds to NICE or reliable health policy sources.