What is the osmolarity of infusion drugs?
The osmolarity of infusion drugs refers to the concentration of solute particles (ions or molecules) in a solution, typically expressed in terms of osmoles per liter (osmol/L). Osmolarity is a measure of the solution's osmotic pressure and is used to determine the number of solute particles in a solution. In the context of infusion drugs, the osmolarity is important for several reasons:
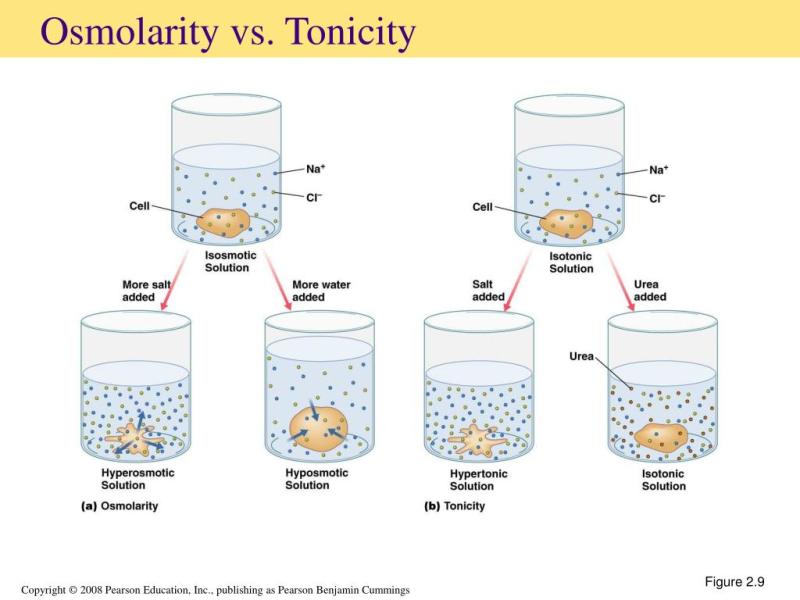
Compatibility with Blood: Intravenous (IV) infusion drugs must be close in osmolarity to blood to prevent damage to red blood cells and ensure safe administration. If a solution is too hypertonic (high osmolarity) or hypotonic (low osmolarity) compared to blood, it can cause adverse reactions.
Fluid Balance: The osmolarity of infusion drugs can influence fluid balance in the body. For example, hypertonic solutions can draw water out of cells, potentially leading to dehydration, while hypotonic solutions may cause cells to swell due to an influx of water.
Drug Efficacy: The osmolarity of a drug can affect its absorption and distribution in the body. Solutions with extremely high or low osmolarity may impact drug effectiveness.
Risk of Complications: If infusion drugs with inappropriate osmolarity are administered, they can lead to complications such as phlebitis (inflammation of veins), hemolysis (rupture of red blood cells), or edema (fluid accumulation).
Healthcare professionals, including pharmacists and nurses, carefully consider the osmolarity of infusion drugs when preparing and administering them to patients. They ensure that the osmolarity of the drug is appropriate for the patient's condition and that it is administered at the correct rate to maintain fluid and electrolyte balance.
It's important for healthcare providers to follow established guidelines and standards for selecting and administering infusion drugs to minimize the risk of osmolarity-related complications and ensure patient safety. Patients receiving infusion therapy should also be informed about the drugs being administered, potential side effects, and the importance of notifying their healthcare provider if they experience any adverse reactions during the infusion.
Osmolarity of Infusion Drugs: What You Need to Know
Osmolarity is a measure of the concentration of dissolved particles in a solution. In the context of infusion therapy, osmolarity is important because it affects the movement of fluids across cell membranes. A solution with high osmolarity will draw fluids out of cells, while a solution with low osmolarity will cause fluids to move into cells.
Calculating Osmolarity for Safe Infusion Drug Administration
The osmolarity of a solution can be calculated using the following formula:
Osmolarity (mOsm/L) = (Number of moles of solute) / (Volume of solution in liters)
For example, a solution that contains 1 mole of sodium chloride (NaCl) in 1 liter of water has an osmolarity of 300 mOsm/L.
Common Infusion Drugs and Their Osmolarity Levels
The osmolarity of infusion drugs can vary widely. Some common infusion drugs and their osmolarity levels are shown in the table below:
| Drug | Osmolarity (mOsm/L) |
|---|---|
| Normal saline | 300 |
| Lactated Ringer's solution | 275 |
| Dextrose 5% in water (D5W) | 254 |
| Dextrose 10% in water (D10W) | 508 |
| Mannitol 20% | 1840 |
The Importance of Osmolarity Matching in IV Therapy
The osmolarity of an infusion drug should be matched as closely as possible to the osmolarity of the patient's plasma. If the osmolarity of the infusion drug is much higher than the osmolarity of the patient's plasma, fluids will be drawn out of cells, which can lead to cellular dehydration and hemolysis (destruction of red blood cells). Conversely, if the osmolarity of the infusion drug is much lower than the osmolarity of the patient's plasma, fluids will move into cells, which can lead to cellular swelling and rupture.
Monitoring and Adjusting Osmolarity in Infusion Therapy
The osmolarity of the patient's plasma should be monitored regularly during infusion therapy. If the patient's osmolarity starts to deviate significantly from normal, the osmolarity of the infusion drug may need to be adjusted.
In addition to monitoring the patient's osmolarity, the nurse should also monitor the patient for signs and symptoms of hemolysis or cellular swelling. These signs and symptoms can include:
- Changes in urine output
- Changes in blood pressure
- Changes in mental status
- Hemoglobinuria (red blood cells in the urine)
Summary
Osmolarity is an important factor to consider when administering infusion drugs. The osmolarity of the infusion drug should be matched as closely as possible to the osmolarity of the patient's plasma to avoid complications such as cellular dehydration, hemolysis, and cellular swelling. The patient's osmolarity should be monitored regularly during infusion therapy, and the osmolarity of the infusion drug should be adjusted as needed.