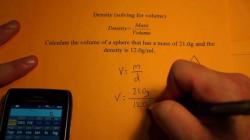How does gram equal to how many liters?
The relationship between grams and liters depends on the density of the substance. The key factor in converting grams to liters is the density, which represents the mass per unit volume. The formula for this relationship is:
Here's a breakdown of the formula:
- Volume (Liters): The result you want to find, representing the volume of the substance in liters.
- Mass (Grams): The initial quantity you have in grams.
- Density (g/mL or g/cm³): The density of the substance, indicating the mass per unit volume. This value is substance-specific and can be found in reference materials or online databases.
To convert grams to liters, divide the mass in grams by the density in grams per milliliter (g/mL) or grams per cubic centimeter (g/cm³), depending on the units used for density.
Here's a step-by-step guide:
Determine the Density:
- Find the density of the substance in either grams per milliliter (g/mL) or grams per cubic centimeter (g/cm³). This information is often provided in textbooks, online databases, or on the product label.
Use the Formula:
- Apply the formula mentioned earlier:
Perform the Calculation:
- Divide the mass in grams by the density to get the volume in liters.
Example:
- Let's say you have 120 grams of a substance with a density of 1.5 g/mL.
- The result would be 80 liters.
Remember to use the correct density value for the specific conditions under which you are working, as density can change with temperature and pressure. Additionally, ensure that your units are consistent, especially if the density is given in grams per cubic centimeter (g/cm³).
Unit conversion inquiry: How does gram equal to how many liters?
The gram and the liter are both units of measurement, but they measure different things. The gram is a unit of mass, while the liter is a unit of volume. This means that it is not possible to directly convert grams to liters.
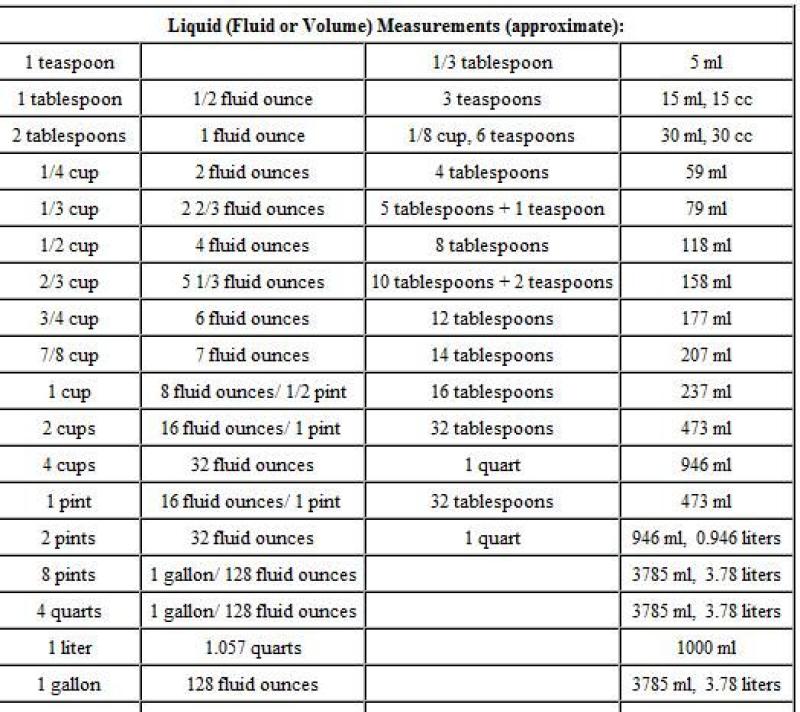
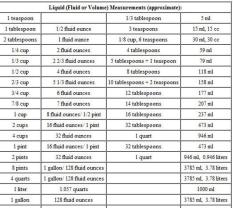
However, it is possible to convert grams to liters if you know the density of the substance you are measuring. Density is a measure of how much mass is contained in a given volume. The density of water, for example, is 1 g/mL. This means that 1 gram of water has a volume of 1 mL.
To convert grams to liters, you can use the following formula:
liters = grams ÷ density
Where:
litersis the volume in litersgramsis the mass in gramsdensityis the density of the substance in grams per liter (g/L)
For example, to convert 100 grams of water to liters, you would use the following calculation:
liters = 100 g ÷ 1 g/L
liters = 100 L
Understanding the conversion between grams and liters and the factors involved
The conversion between grams and liters is based on the density of the substance being measured. Density is a measure of how much mass is contained in a given volume. It is typically expressed in units of grams per liter (g/L) or grams per milliliter (g/mL).
The density of a substance can vary depending on the temperature and pressure at which it is measured. For example, the density of water is 1 g/mL at 4 degrees Celsius and 1 atm of pressure. However, the density of water will decrease if the temperature is increased or the pressure is decreased.
The conversion between grams and liters is only accurate if you know the density of the substance you are measuring. If you do not know the density of the substance, you will not be able to accurately convert between grams and liters.
Tips for students and scientists in converting measurements between mass and volume units
Here are some tips for students and scientists in converting measurements between mass and volume units:
- Use a density reference table. A density reference table will give you the density of a wide variety of substances.
- Be careful when measuring mass and volume. Mass and volume can be difficult to measure accurately, so it is important to be as precise as possible.
- Use the correct units. Make sure that you are using the correct units for mass, volume, and density.
- Double-check your calculations. It is always a good idea to double-check your calculations to make sure that you are getting the correct answer.